Influenza virus infection is characterized by symptoms ranging from mild congestion and body aches to severe pulmonary edema and respiratory failure. While the majority of those exposed have minor symptoms and recover with little morbidity, an estimated 500,000 people succumb to IAV-related complications each year worldwide. In these severe cases, an exaggerated inflammatory response, known as “cytokine storm”, occurs which results in damage to the respiratory epithelial barrier and development of acute respiratory distress syndrome (ARDS). Data from retrospective human studies as well as experimental animal models of influenza virus infection highlight the fine line between an excessive and an inadequate immune response, where the host response must balance viral clearance with exuberant inflammation. Current pharmacological modulators of inflammation, including corticosteroids and statins, have not been successful in improving outcomes during influenza virus infection. We have reported that the amplitude of the inflammatory response is regulated by Linear Ubiquitin Assembly Complex (LUBAC) activity and that dampening of LUBAC activity is protective during severe influenza virus infection. Therapeutic modulation of LUBAC activity may be crucial to improve outcomes during severe influenza virus infection, as it functions as a molecular rheostat of the host response. Here we review the evidence for modulating inflammation to ameliorate influenza virus infection-induced lung injury, data on current anti-inflammatory strategies, and potential new avenues to target viral inflammation and improve outcomes.
La infección por el virus de la gripe se caracteriza por síntomas que van desde la congestión leve y los dolores corporales hasta el edema pulmonar grave y la insuficiencia respiratoria. Aunque que la mayoría de las personas expuestas presentan síntomas leves y se recuperan con poca morbilidad, se estima que cada año 500.000 personas en todo el mundo fallecen por las complicaciones relacionadas con esta infección. En estos casos graves, se produce una respuesta inflamatoria exagerada, conocida como «tormenta de citocinas», que causa daños en la barrera epitelial respiratoria y el desarrollo del síndrome de distrés respiratorio agudo. Los datos de estudios retrospectivos en humanos, así como de modelos animales experimentales de infección por el virus de la gripe, resaltan la delgada línea que existe entre una respuesta inmunitaria excesiva y una inadecuada, cuando la respuesta del huésped debe mantener el equilibrio entre el aclaramiento viral y la inflamación exagerada. Los moduladores farmacológicos de la inflamación actuales, incluidos los corticoides y las estatinas, no han tenido éxito a la hora de mejorar los resultados de la infección por el virus de la gripe. Hemos publicado que la amplitud de la respuesta inflamatoria está regulada por la actividad del complejo de ensamblaje de cadenas lineales de ubiquitina (LUBAC, por sus siglas en inglés) y que la atenuación de la actividad de LUBAC protege durante la infección grave por este virus. La modulación terapéutica de la actividad de LUBAC puede ser crucial para mejorar los resultados, ya que funciona como un reóstato molecular de la respuesta del huésped. Aquí revisamos la evidencia al respecto de la modulación de la inflamación para mejorar el daño pulmonar inducido por la infección por el virus de la gripe, los datos sobre las estrategias antiinflamatorias actuales y las posibles nuevas vías para tratar la inflamación viral y mejorar los resultados.
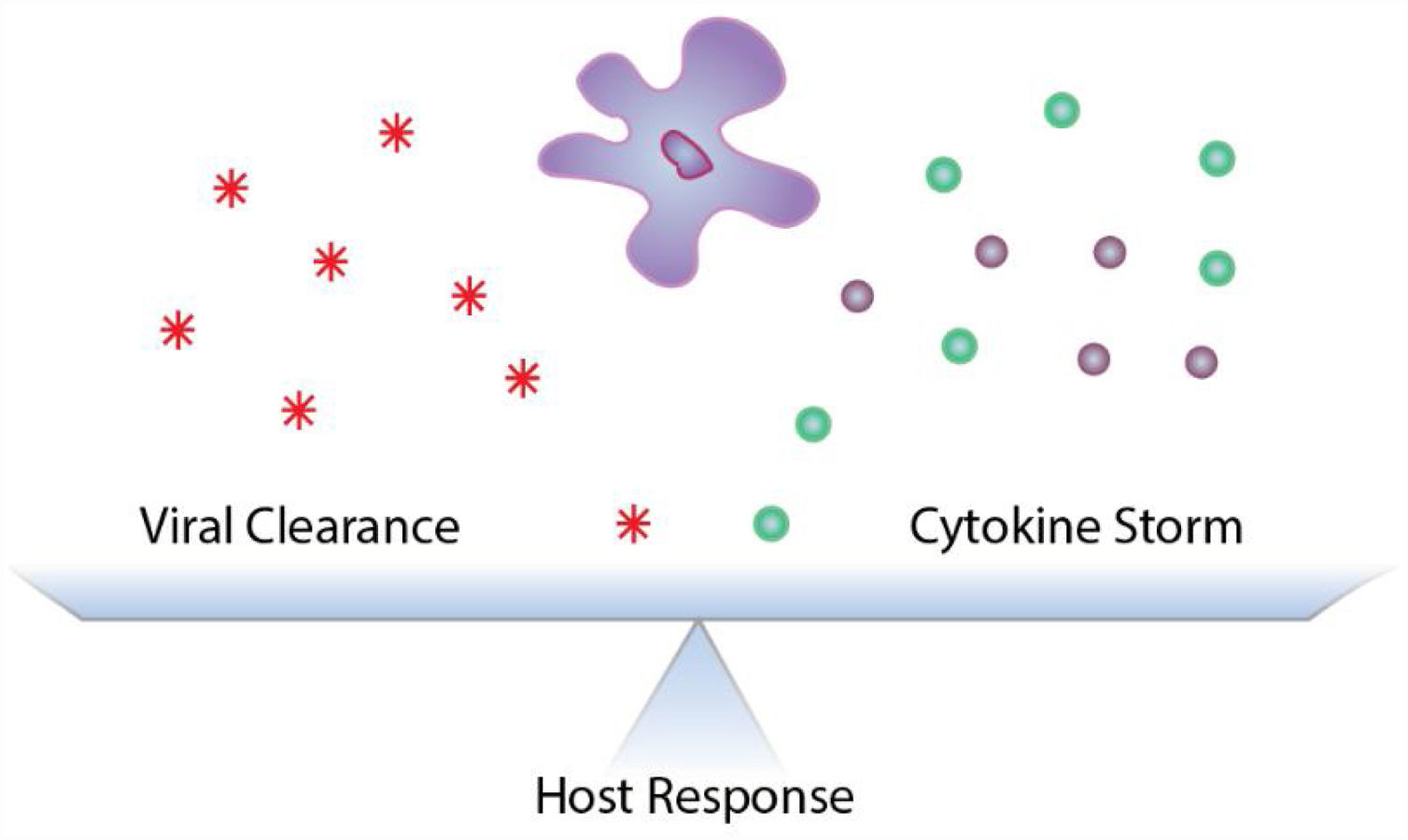
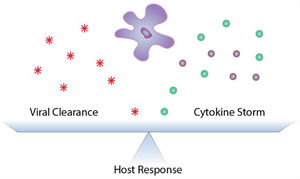
Seasonal influenza A viral infection affects a significant proportion of the population worldwide, with an estimated 500,000 people succumbing to IAV-related complications each year. While most patients infected with influenza A virus (IAV) recover without major sequelae, severe viral pneumonia is one of the most common causes of acute respiratory distress syndrome (ARDS).1–4 Clinically, ARDS presents with bilateral pulmonary infiltrates, hypoxemia, pulmonary edema and widespread lung inflammation that lead to high mortality rates due respiratory and to multiple organ failure.1,4–6 ARDS patients can be sub-grouped based on the severity of the inflammatory response, where patients with hyper-inflammation have worse clinical outcomes, spending more days on mechanical ventilation, experiencing increased incidence of organ failure and a higher mortality rate compared to hypo-inflammatory ARDS patients.7 Impairment of gas exchange in IAV-induced ARDS, in large part, is due to damage to the respiratory epithelial barrier and edema accumulation.1,4–8 During IAV infection, an exaggerated inflammatory response, known as “cytokine storm”, can occur leading to the development of hyper-inflammatory ARDS, increasing IAV-induced morbidity and mortality (Fig. 1).4 Post-mortem studies of lungs from IAV-infected patients show extensive diffuse alveolar damage characterized by edema, cellular infiltration, thickening of alveolar walls, and necrosis.9 Interestingly, a study of critically ill patients showed no differences in pulmonary viral load between those who died and those who recovered, while mortality directly correlated with exuberant inflammation, further supporting a maladaptive host response as the major driver of IAV-induced lung injury.10–14 Similar observations are being reported in patients with severe coronavirus disease (COVID-19), where severe lung damage is associated with increased pro-inflammatory cytokines and respiratory failure from ARDS is the leading cause of mortality.15,16 With no virus-specific treatment options currently validated, therapies which target the inflammatory response are currently being considered for patients with severe COVID-19.17,18 However, as it has been reported for severe IAV infections, current anti-inflammatory drugs have pleiotropic effects and may lack the specificity needed to carefully calibrate the host response.
Respiratory epithelial cells, as primary targets for IAV infection and replication, initiate inflammatory signaling.19–22 In response to respiratory epithelial derived cytokines, innate immune cells, such as neutrophils, monocyte-derived inflammatory macrophages, and natural killer (NK) cells are recruited to the airspace.23 Together with tissue resident alveolar macrophages, recruited innate immune cells are critical for control of viral replication through both lysis and clearance of virus-infected cells.23–26 However, in addition to controlling viral spread, innate immune cells contribute to the overproduction of pro-inflammatory cytokines that enhance IAV-induced lung injury (Fig. 2). The pulmonary immune response must be carefully balanced, simultaneously promoting viral clearance and limiting excessive inflammation to maintain proper lung function.
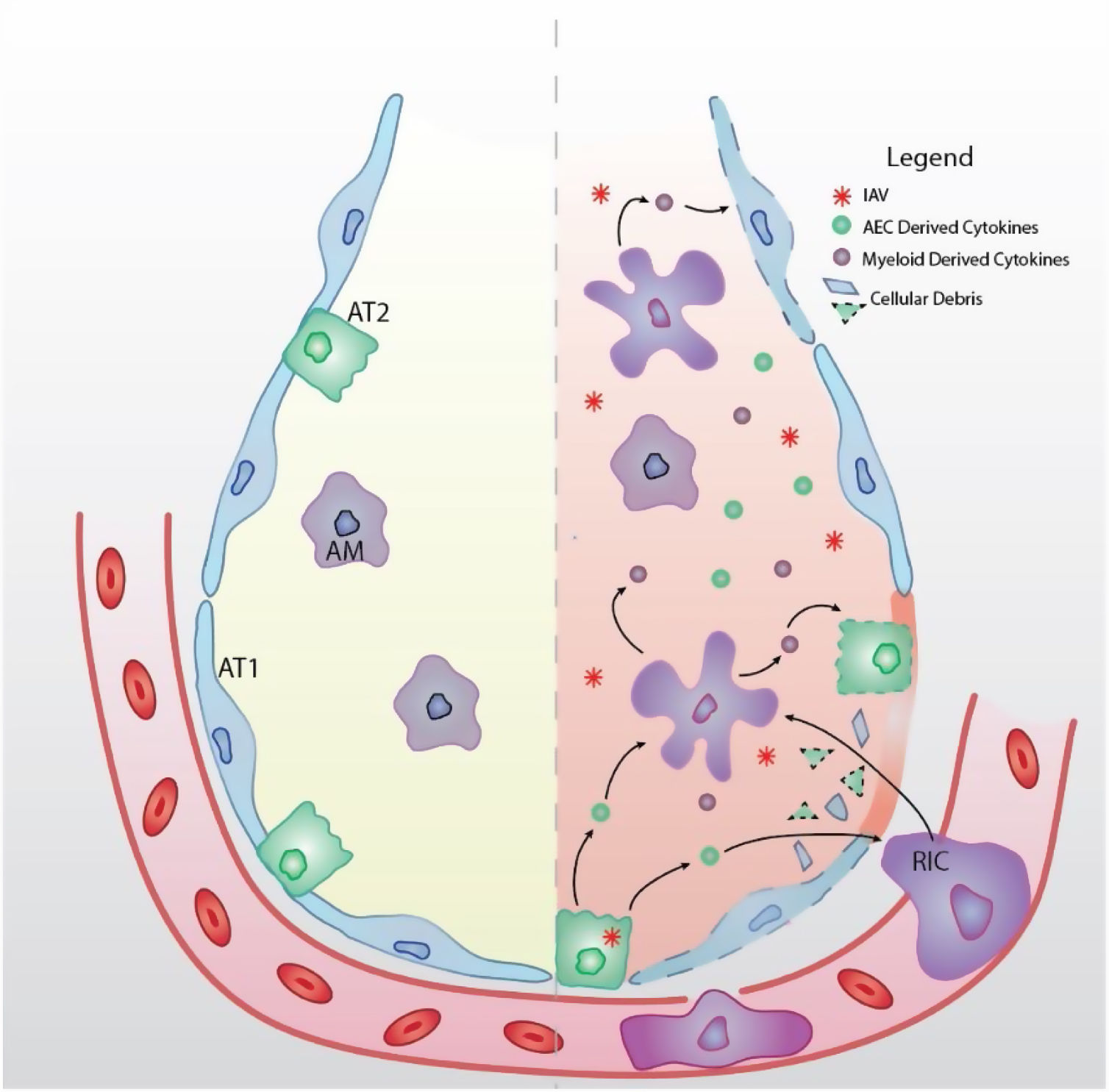
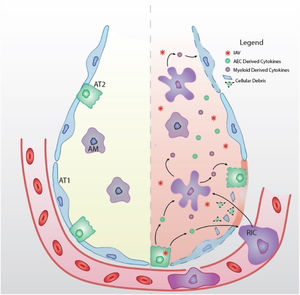
Schematic model of IAV-induced ARDS (Left) The healthy alveolus is free of fluid with tight junctions between alveolar type 1 (AT1) and type 2 (AT2) epithelial cells. Tissue resident alveolar macrophages (AM) facilitate lung homeostasis through clearance of cellular and environmental debris. (Right) Upon inhalation, IAV targets alveolar epithelial cells (AEC) for infection and replication. IAV infection induces epithelial cytokine production that recruits immune cells to the airspace. Recruited immune cells (RIC) contribute to hyper-inflammatory environment and which further contributes to cytokine storm and tissue damage (dashed outline). Barrier disruption due to the denuded epithelium allows extravasation of proteinaceous edema fluid into the airspace that disrupts gas exchange and causes ARDS.
Findings from animal models of IAV infection have shown that modulation of the host immune response is associated with reduced lung injury and improved survival.11,14,27,28 Blockade of specific immune cell subsets has been shown to improve outcomes in mouse models of severe IAV infection. For example, genetic deletion of the chemokine receptor CCR2 inhibited the recruitment of monocyte-derived inflammatory macrophages during IAV infection and resulted in reduced lung injury with improved survival. However, loss of this myeloid cell population resulted in a delay in viral clearance.29,30 Moreover, adoptive transfer of NK cells from IAV-infected lungs, as compared to NK cell from naïve lungs, resulted in increased mortality of influenza-infected mice,31 suggesting that inflammation-dependent activation, rather than recruitment, drives the observed pathology. Taken together data from these animal models suggest that the determinant of influenza severity be orchestrated by respiratory epithelial cells.
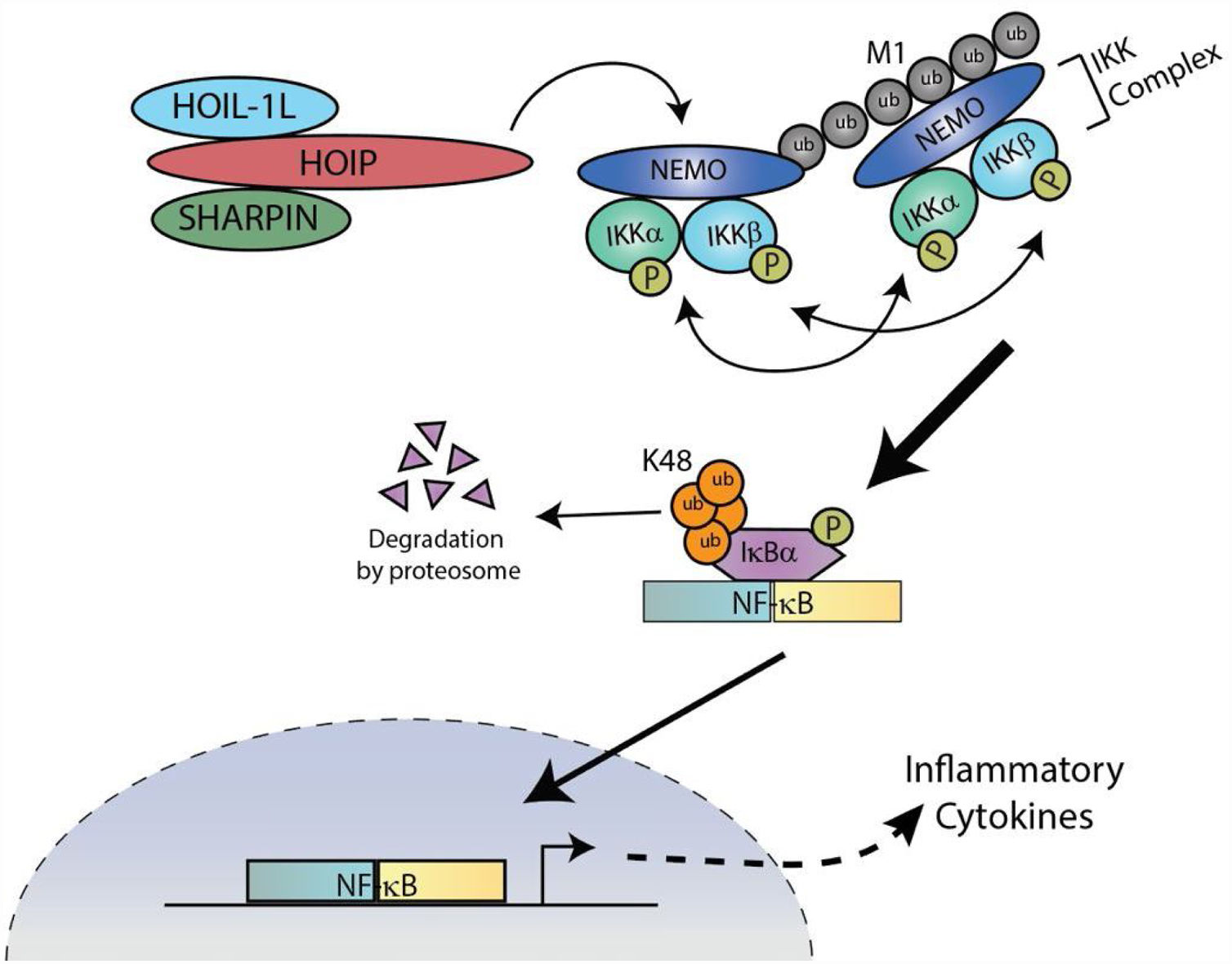
LUBAC regulates the amplitude of the lung epithelial driven responses during IAV infectionThe linear ubiquitin assembly complex (LUBAC) is a multi-protein E3 ubiquitin ligase complex composed of two stabilizing proteins, the Heme-Oxidized Iron responsive element binding protein 2 ubiquitin Ligase-1L (HOIL-1L) and Shank-Associated RH domain-Interacting Protein (SHARPIN), and a catalytic component, HOIL-1-Interacting Protein (HOIP)32–34 (Fig. 3). The proteins within the heteromeric complex contain multiple domains for interactions within the complex, ubiquitin binding, as well as catalytic activity.32–36 LUBAC is an essential regulator of NF-κB activation and has been shown to act as a molecular rheostat, regulating the amplitude of the epithelial-driven inflammatory response dung IAV infection.35,36
LUBAC is necessary for NF-κB dependent inflammation
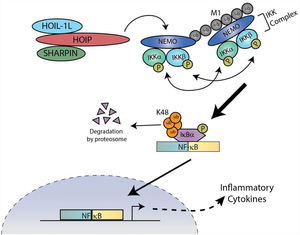
LUBAC covalently attaches linear ubiquitin chains to NEMO, which facilitates the recruitment of additional IKK complexes. Stably docked IKK complexes result in the efficient transautophosphorylation and activation of proximal IKKα/β, followed by the phosphorylation and degradation of IκBα. NF-κB translocates to the nucleus to stimulate transcription of inflammatory genes.
The respiratory epithelium actively participates in the first line of defense against pathogens by orchestrating host innate immunity.23,37–39 As IAV replicates within the respiratory epithelium cells, the cytosolic pattern recognition receptor (PRR), RIG-I, is activated and initiates formation of a signaling platform to which LUBAC is recruited. LUBAC covalently attaches Met-1 linked linear ubiquitin chains to the NF-κB essential modulator (NEMO), a component of the inhibitor of NF-κB (IκB) kinase (IKK) complex along with IKKα and IKKβ.40,41 Due to the high affinity of NEMO's ubiquitin binding domain for linear chains, linear ubiquitination of NEMO facilitates the recruitment of additional IKK complexes, which results in the efficient trans-autophosphorylation and activation of proximal IKKβ followed by the phosphorylation and degradation of IκBα and robust NF-kB activation (Fig. 3).34,35,40,42,43
Recent reports show that destabilization of respiratory epithelial LUBAC, via loss of the non-catalytic component HOIL-1L, dampens the host response during severe influenza and promotes survival with reduced lung injury as well as reduced viral titers. However, when LUBAC activity is abolished through deletion of HOIP, the alveolar epithelia driven inflammatory response is inhibited and mortality is increased. These findings highlight the fine line between an excessive and an inadequate immune response and suggest that therapeutic modulation of LUBAC activity may be crucial, as it functions as a rheostat regulating the amplitude of the host response to IAV infection.
LUBAC covalently attaches linear ubiquitin chains to NEMO, which facilitates the recruitment of additional IKK complexes. Stably docked IKK complexes result in the efficient transautophosphorylation and activation of proximal IKKα/β, followed by the phosphorylation and degradation of IκBα. NF-κB translocates to the nucleus to stimulate transcription of inflammatory genes.
Pharmacological immunomodulation during IAV infectionCurrent anti-influenza strategies are limited to yearly vaccination or administration of antiviral drugs, however, short therapeutic windows, viral mutation, and resistance to current therapies limit their effectiveness. Despite available vaccination and anti-viral drugs, the most recent pandemic in 2009 resulted in an estimated 151,700–575,400 deaths in its first year of circulation worldwide.44 The pandemic strain contained a novel assortment of viral genes not previously identified in animal or human populations. From its first detection in April 2009, it was only 3 months until resistance to anti-viral drugs was reported, and it took an additional 3 months before the first vaccine offering protection from the pandemic strain was administered.45 In addition to emerging pandemic strains, between 291,000 and 646,000 people worldwide die from seasonal influenza-related respiratory illnesses each year.46 Novel mutations and reassortments of the virus will inevitably lead to the next IAV pandemic; therefore, the use and development of therapeutics that target conserved host pathways, rather than the virus itself, hold promise to curtail the impact of viral infection. Moreover, a heterogeneous response to IAV with the same virulence exists within the population, suggesting that host factors play a crucial role regulating the host response and determining the severity of lung injury.2,3,47 Additionally, experimental evidence from human studies and animal models of severe IAV show that viral titers do not always correlate with severity of disease, but rather ARDS induced “cytokine storm” is the major driver of morbidity and mortality.4,28,38,48
Severe IAV infection is associated with inflammatory cytokines in humans and mice. Due to their pleiotropic and redundant effects, targeting of individual cytokines may not be a suitable approach to reduce pathology during IAV infection. Instead, dampening of the immune response may be more effective, as was the case with LUBAC destabilization noted above.36 FDA-approved anti-inflammatory drugs, including corticosteroids and statins, have been proposed for the treatment of “cytokine storm” associated with severe IAV infection.49 Moreover, current data regarding their efficacy is limited to mouse models and retrospective patient observations.49,50 Corticosteroids have been shown to be effective in limiting the inflammation in some lung pathologies.49–51 However, observational studies of the impact of corticosteroid treatment of IAV-infected patients suggest against their use; with administration associated with higher incidence of hospital-acquired pneumonia, longer duration of mechanical ventilation, and increased mortality.50 Similarly, clinical evidence does not support corticosteroid treatment for COVID-19 lung injury.52 Statins are another class of drugs recognized for their ability to dampen inflammation.49 While experimental evidence from mouse models using statins during IAV infection have been inconclusive regarding their benefit,49,53,54 retrospective analysis of patient data suggests an association between statin treatment and lower IAV mortality rates.55 These examples highlight the need for new avenues of drug discovery and validations, as no currently available immune modulators have convincingly demonstrated their ability to improve outcomes during influenza infection.
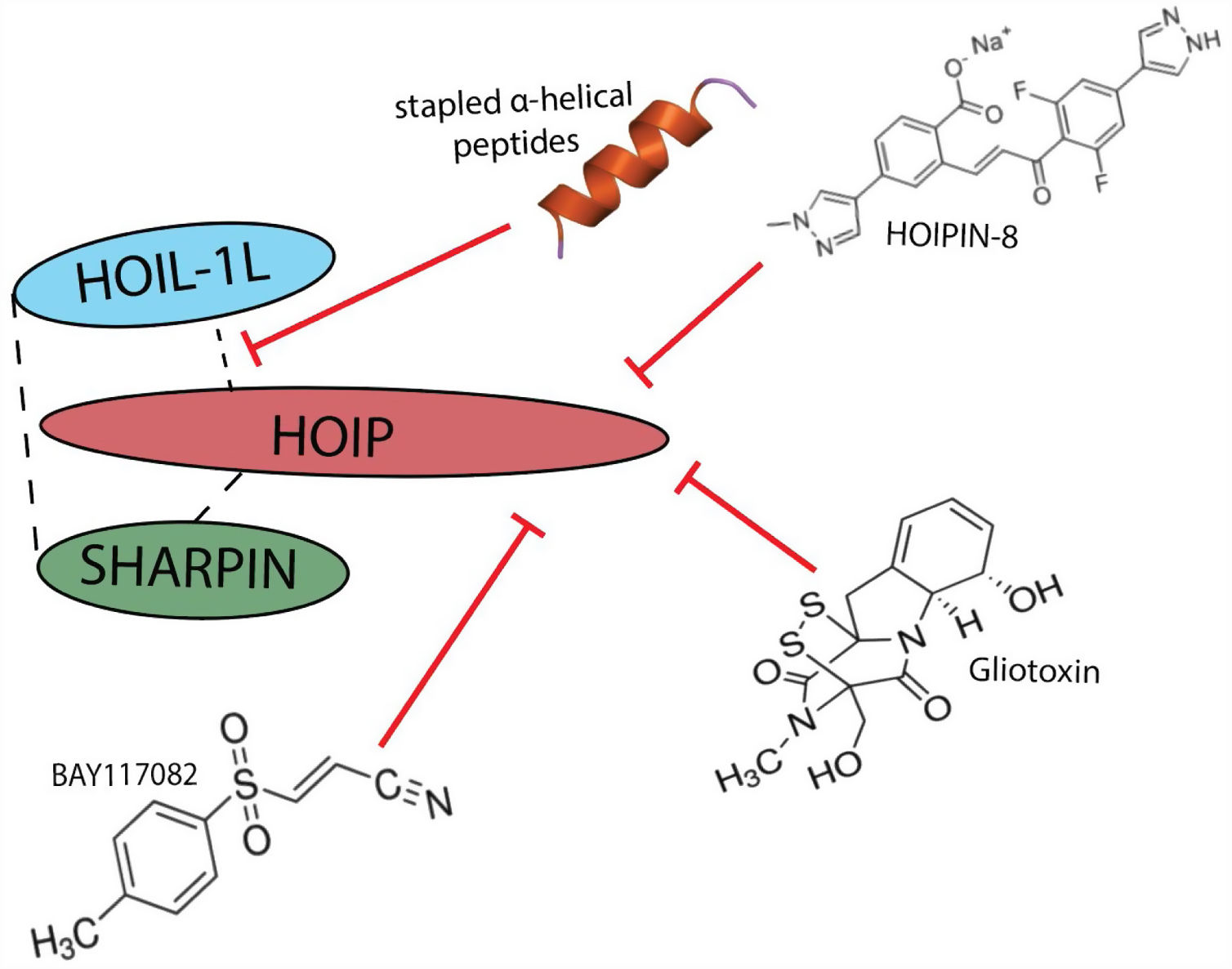
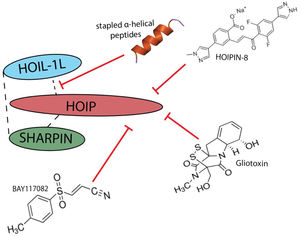
LUBAC represents a potential new target for limiting the pathological inflammation that occurs during IAV infection. Several chemical inhibitors as well as peptides that bind HOIP have been used to inhibit LUBAC activity in cell culture56–59 and in vitro assays60,61 and support the specific targetablility of LUBAC (Fig. 4). Currently, LUBAC inhibitors fall into two categories: those that target the catalytic activity of HOIP (i.e. BAY117082, Gliotoxin, HOIPIN) or those that disrupt the interaction between LUBAC components to destabilize the complex (i.e. stapled peptides). BAY117082, a small molecule commonly used as an inhibitor of NF-κB activation. It has been observed that treatment of RAW 264.7 macrophages with BAY117082 prevented IL-1 stimulated formation of linear ubiquitin chains. Further investigation revealed that BAY117082 irreversibly inhibits LUBAC through a chemical reaction with cysteine residues in the active site of HOIP, the catalytic unit of LUBAC.58 While BAY117082 represents a potent inhibitor of LUBAC activity, it targets multiple components of the ubiquitin system, including inhibition of E2 ubiquitin conjugating enzymes, and possible proteasome inhibition.58 As such, use of BAY117082 is not suitable for the study of LUBAC-dependent physiological functions or therapeutic targeting of LUBAC activity in disease. Gliotoxin, a fungal metabolite, was identified in a high-throughput screening for LUBAC inhibitors using a time-resolved FRET-based screening system. While gliotoxin is known to have multiple cellular targets, it is able to inhibit LUBAC activity and downstream activation of NF-κB at 10x lower concentrations.61 Gliotoxin's strong, irreversible binding to the catalytic site of HOIP makes it a selective inhibitor of LUBAC activity.61 Interestingly, the potency of gliotoxin has been shown to vary between cell types, with myeloid and lymphoid cells being more sensitive to gliotoxin-mediated NF-κB inhibition than epithelial cells.61,62 However, this irreversible inhibition may quench the inflammatory response and increase susceptibility to secondary infections. HOIPINs are synthetic small molecules that reversibly inhibit LUBAC though targeting of HOIP activity, displaying both LUBAC specificity as well as low cytotoxicity. Several derivatives have been made with varying degrees of efficacy (HOIPIN-1-8), with HOIPIN-8 showing significantly enhanced ability to prevent LUBAC-mediated NF-κB activation in response to TNF-α without cytotoxicity compared to the other derivatives in vitro.57 Conversely, stapled α-helical peptides developed based on specific LUBAC structures disrupt interactions necessary for stable complex formation.56,63 Stapled peptides are a class of synthetic macrocycles where the secondary α-helix structure is stabilized by the introduction of a hydrophobic bridge or “staple” that rigidifies specific areas to inhibit protein:protein interactions.56 Stapled peptides based on the HOIP ubiquitin binding domain have been shown to successfully inhibit LUBAC activity in vitro by disrupting its interaction with HOIL-1L and destabilizing the overall complex.56,63 While several inhibitors of LUBAC have been developed and shown promise in vitro, no data is available detailing their efficacy in vivo. Further investigation in to these compounds which target LUBAC stability to modulate the degree of LUBAC activity is warranted as they may be therapeutically beneficial for the treatment of hyper-inflammatory response during viral infection, where a graded host response is necessary.
ConclusionsWithin the past 150 years, IAV has been the causative agent of at least five pandemics (1889, 1918, 1957, 1968, 2009).47,64 In addition to IAV, novel viral threats, such as the coronavirus outbreaks of 2003, 2015 and 2019, quickly spread worldwide before virus-specific vaccines or pharmacological options could be developed. Thus, therapies that target conserved host pathways may provide a novel universal treatment strategies, regardless of viral sequence. Findings from animal models of IAV infection have shown that inhibition of the exuberant host immune response is associated with reduced lung injury and improved survival.11,14,27,28 However, current FDA-approved anti-inflammatory drugs, such as corticosteroids and statins, have failed to show benefit during severe IAV infection.49,50 Beyond viral infections, the amplitude of the inflammatory response has been shown to be a critical determinant in outcome during bacterial sepsis in community acquired pneumonia, a common complication post IAV infection.51,65 Analysis of a cohort of patients showed that reductions in the inflammatory response during bacterial pnumonia, both due to host inability to mount a response or the administration of anti-inflammatory steroids, lead to increased mortality.51,65 These clinical observations highlight the need to balance the inflammatory response during viral infection, not only to improve lung injury during the primary viral infection, but also to prevent poor outcomes to secondary infections.
In addition to the seasonal threat of influenza, we must also be cautious in the regulation of inflammation in treatment of the ongoing CoVID-19 pandemic. While a subgroup of patients with severe COVID-19 develop ‘cytokine storm’,15,16,18 it must be remembered that current anti-inflammatory drugs have pleiotropic effects and lack the specificity needed to carefully calibrate the host response. As such, newly developed pharmacologics such as those that target LUBAC, a molecular rheostat of inflammatory signaling, have the potential to fine tune inflammation and moderate the host response. Further investigation of compounds which modulate LUBAC activity is warranted, as they may be therapeutically beneficial for the treatment of hyper-inflammatory response during viral infections, where a milder host response should improve outcomes.
Conflict of interestThe authors have declared that no conflict of interest exists.