An electronic nose is a non-invasive device that uses a series of built-in electronic chemical sensors to identify volatile organic components (VOCs) in exhaled air1,2. Previous studies using this technology have determined specific breathprints in patients with chronic respiratory diseases such as chronic obstructive pulmonary disease (COPD)3, asthma4, and cystic fibrosis5. The value of VOC analysis in distinguishing patients with diffuse ILD6 from healthy controls has also been recently recognized. Our aim, therefore, was to determine the usefulness of the electronic nose in distinguishing not only ILD patients from healthy controls, but also from individuals with COPD.
To this end, we conducted a prospective and observational exploratory study between 2016 and 2018. The study protocol was approved by the ethics and research committee of our institution (IIBSP-COL-2015-40). Informed consent was obtained from all participants prior to inclusion in the study. Subjects with diffuse ILD attending specialist clinics were included. The COPD group and the control group are from 2 cohorts, also prospective, that were selected in previous studies conducted by our group7,8. The inclusion criteria of the ILD group were: patients older than 18 years; diagnosis of ILD according to the current clinical guidelines of the Spanish Society of Pulmonology and Thoracic Surgery9; and no contraindication for participation in the study. Exclusion criteria were: presence of other chronic or acute lung diseases; and no chest CT or lung function tests.
To evaluate the VOC profile, exhaled air was collected according to the methodology described elsewhere4,5,8,10. Essentially, the subject breathes at tidal volume for 3 min and a breath sample is collected in a Tedlar® bag using a Hans Rudolph valve fitted with an inspiratory filter and an expiratory silica reservoir to dry the expired air. In the 12 h prior to the test, all patients had to remain in fasting, refrain from smoking, and suspend any inhaled medication. The electronic nose (Cyranose 320®, Smith Detections, Pasadena, CA) uses an array of 32 sensors to generate an exhaled VOC profile, known as a breathprint.
The breathprints of patients included in the study were compared with a pattern recognition application on MATLAB software (v.R2012a). The results were shown as 1- or 2-dimensional graphs using previously published logistic regression algorithms8,10. Principal component analysis (PCA) was used to reduce raw data to 3 major factors. These PCA factors were analyzed by ANOVA univariate analysis, followed by the least significant difference post-hoc test. Patients were classified based on PCA factors using a linear canonical discriminant analysis, calculated as the one that obtained the best percentage of correctly classified patients. The discriminant function was trained with samples using the leave-one-out method, and the samples of the left-out subject were then analyzed. If 3 or 4 samples are correctly assigned, the discriminant function is considered valid for that individual. The process was repeated for all individuals and the results were used to calculate the cross-validated accuracy value (%)3. A p-value <0.05 for the trained discriminant function was considered statistically significant. Finally, a receiver operating characteristics (ROC) curve was generated using the discriminant function results, combining all samples from a single subject. The area under the ROC curve (AUROC) was calculated by multiple logistic regression.
A total of 130 subjects were included in the study analysis, distributed as follows: 59 patients (45%) in the ILD group, 40 patients (31%) in the COPD group, and 31 patients (24%) in the healthy control group. Although the subjects in the ILD and COPD groups were of similar ages (mean of 65 and 68 years, respectively), the control group was younger (42 years). Most healthy controls had no history of smoking (16.1%), while the other 2 groups were comprised mostly of smokers or former smokers (ILD 59.3%, COPD 100%). With regard to lung function, as might be expected, the COPD group showed an obstructive pattern on spirometry (FEV1 41% ± 10 SD) that was not observed in the other 2 groups.
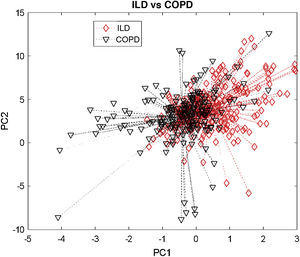
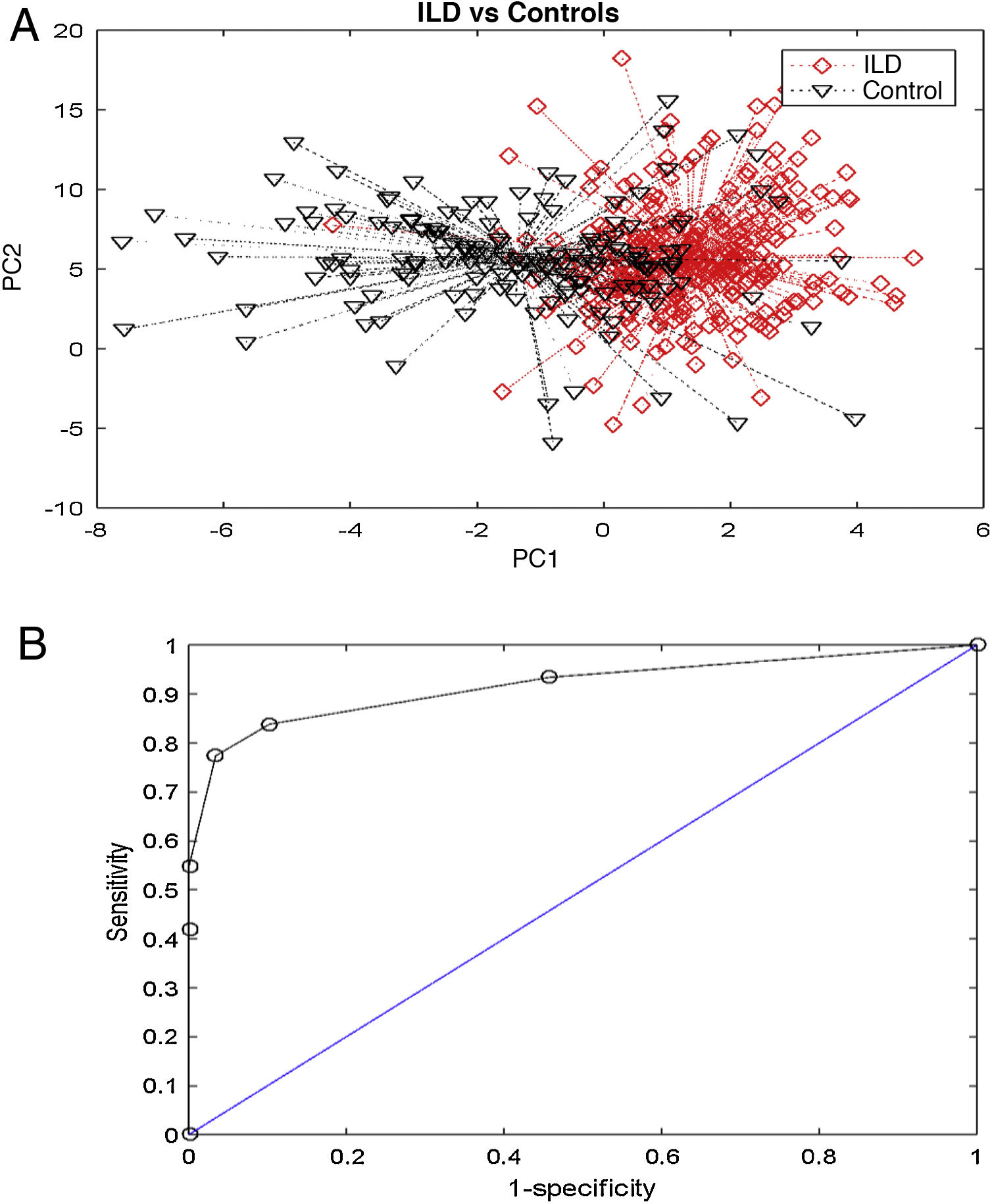
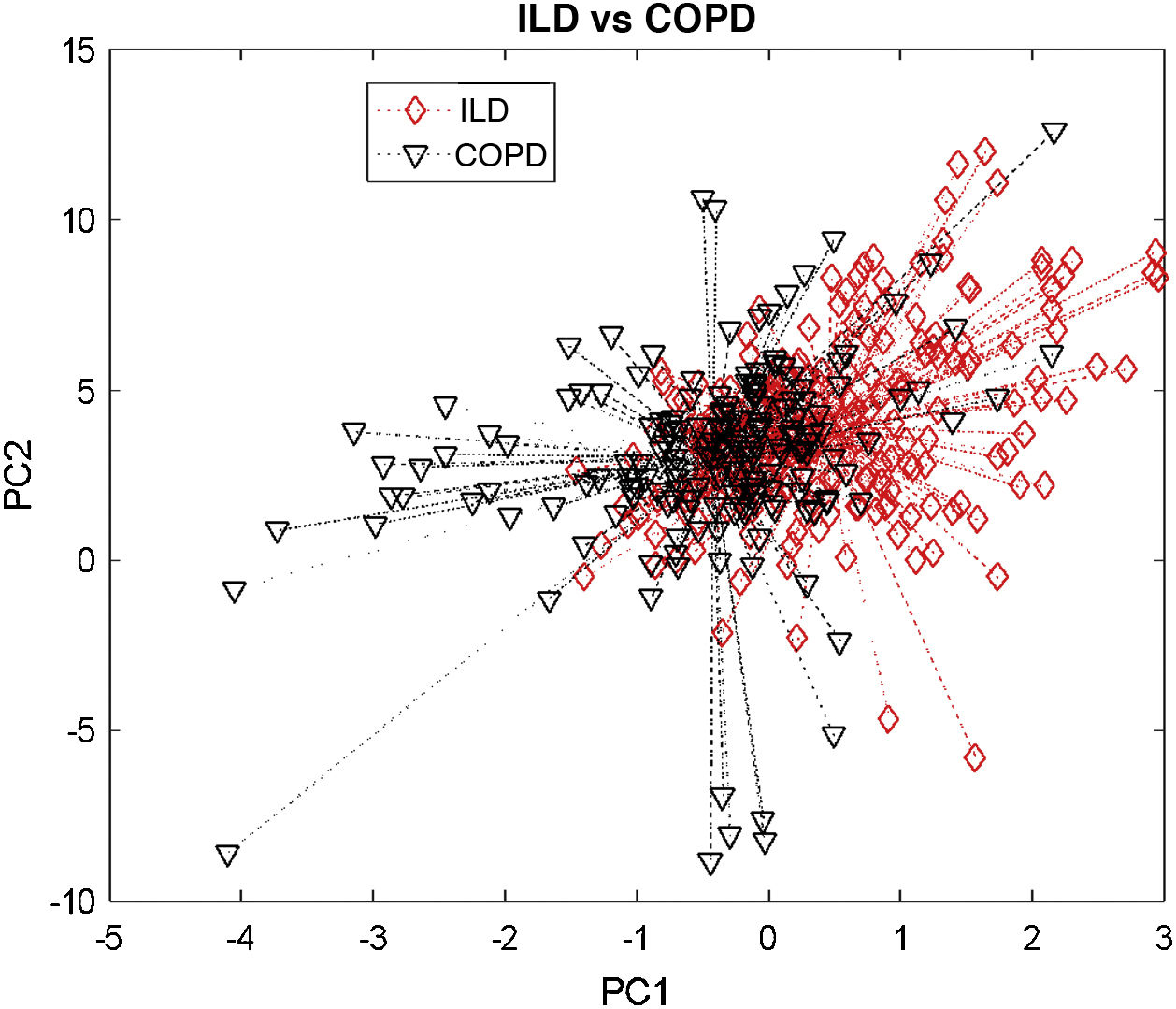
The VOC (breathprint) profile determined by the electronic nose in the ILD group and the control group was clearly different from the visual evaluation (Fig. 1A). Canonical discriminant analysis showed a cross-validation accuracy of 90% (p < 0.001). The ability of the technique to detect patients with ILD according to the ROC analysis was optimal (AUROC 0.92), with a sensitivity of 96.6%, a specificity of 77.4%, a positive predictive value (PPV) of 0.89, and a negative predictive value (NPV) of 0.73 (Fig. 1B). The comparison between the ILD and COPD groups also showed statistically significant results, but this time the accuracy was lower. Canonical discriminant analysis showed a cross-validation accuracy of 66% (p = 0.007) (Fig. 2) and the AUROC value was 0.73.
VOC (breathprint) profile in the ILD and control groups. (A) Principal component analysis showing differences in breathprint between patients with ILD and healthy subjects (90% cross-validation accuracy [p < 0.001]). (B) AUROC curve for the discrimination of ILD subjects according to their breathprint.
Our findings show that the electronic nose could be a useful tool for discriminating between ILD patients and healthy subjects. Only 2 similar studies have been published in the medical literature. The first, by Krauss et al., analyzed various ILDs, including idiopathic pulmonary fibrosis or cryptogenic organizing pneumonia, and compared them with healthy controls11. More recently, Moor et al. compared a larger cohort of subjects with ILD (322) versus healthy controls, obtaining an AUROC of 16. It is important to mention that this study also included a validation cohort, for which the same value was obtained. These results are almost identical to those obtained in our study (AUROC 0.92). An innovative aspect of our research is that it also shows, albeit with less discriminative capacity (66% cross-validation accuracy, AUROC 0.73), that the electronic nose can also distinguish between subjects with ILD and COPD.
The greatest value of this study is that it is the first to prospectively include emerging, i.e. treatment-naïve, cases of ILD (new diagnosis). In contrast, the main limitations are its single-center design and relatively small cohort. Multicenter studies are clearly needed to validate these results. Another possible limitation is the fact that the group of healthy controls is younger than the study group. However, previous studies indicate that neither age, sex, nor smoking have a significant influence on breathprints12–14.
In conclusion, the electronic nose is a non-invasive device that could potentially detect subjects with ILD in the general population and therefore improve the screening process for the disease.
FundingThis research was supported by the Spanish Society of Pulmonology and Thoracic Surgery through the EPID Futuro 2015 grant.
AuthorshipD.C., S.B., and O.S. designed the study. D.C., S.B. and P.M-B were responsible for patient inclusion, J.G. for electronic nose determination, and J.L.M. for volatile particle analysis. All authors participated in the data analysis and writing of the manuscript.
We thank our patients for their altruistic participation in this study.
Please cite this article as: Castillo Villegas D, Barril S, Giner J, Millan-Billi P, Rodrigo-Troyano A, Merino JL, et al. Estudio de la enfermedad pulmonar intersticial difusa mediante el análisis de partículas volátiles en el aire exhalado. Arch Bronconeumol. 2022;58:99–101.


![VOC (breathprint) profile in the ILD and control groups. (A) Principal component analysis showing differences in breathprint between patients with ILD and healthy subjects (90% cross-validation accuracy [p < 0.001]). (B) AUROC curve for the discrimination of ILD subjects according to their breathprint. VOC (breathprint) profile in the ILD and control groups. (A) Principal component analysis showing differences in breathprint between patients with ILD and healthy subjects (90% cross-validation accuracy [p < 0.001]). (B) AUROC curve for the discrimination of ILD subjects according to their breathprint.](https://static.elsevier.es/multimedia/03002896/0000005800000001/v1_202201130549/S0300289621004002/v1_202201130549/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w98FxLWLw1xoW2PaQDYY7RZU=)