The new COVID-19 has become the largest global epidemic in recent centuries. Mortality is influenced by the different complications that patients develop; nevertheless, some of these remain underreported. In May 2020, we observed the first case of spontaneous pneumomediastinum and pneumopericardium associated with COVID-19 infection in a Peruvian public hospital. By July, a total of 19 cases of pneumomediastinum1–10 and only one case of spontaneous pneumopericardium11 have been reported in COVID-19 patients. Therefore, we decided to search for these two complications through imaging studies.
We encountered twelve COVID-19 patients that developed spontaneous pneumomediastinum and/or pneumopericardium during their hospitalization in a Peruvian public hospital between April and July 2020. Pneumomediastinum and pneumopericardium were defined by chest computed tomography (CT). Images were reviewed by a general radiologist and a cardiac imaging specialist. We excluded patients that received previous mechanical ventilation. COVID-19 diagnosis was defined by symptoms and radiologic findings compatible with COVID-19 pneumonia along with a positive COVID-19 rapid test. Confirmatory testing was not available due to limited hospital resources. Eight patients fulfilled the criteria; and a subgroup of four patients without a positive COVID-19 rapid test was also included to increase sample size.
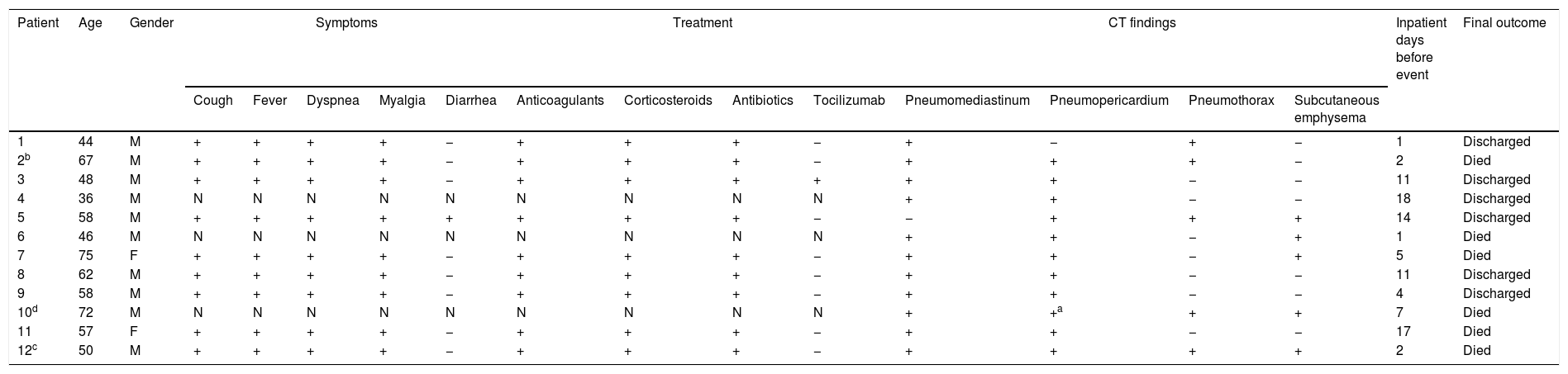
Clinical characteristics and CT findings are presented in Table 1. The median (IQR) age of patients was 57.5 (47–64.5) years; 83% of patients were male. The mean duration of symptoms before hospital admission was 8.7 (3) days. Symptom, comorbidity and treatment information could only be collected in nine patients. All of them had fever, cough, dyspnea and tachypnea on the time of admission; six patients reported thoracic pain during hospitalization. The median of days from the onset of symptoms until thoracic pain appeared was 17. Laboratory findings showed elevated white-cell count and lymphocytopenia in 83% of patients. All patients denied thoracic trauma and previous lung disease. Two patients had diabetes mellitus and one had hypertension and hypothyroidism.
Clinical characteristics and CT findings.
| Patient | Age | Gender | Symptoms | Treatment | CT findings | Inpatient days before event | Final outcome | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cough | Fever | Dyspnea | Myalgia | Diarrhea | Anticoagulants | Corticosteroids | Antibiotics | Tocilizumab | Pneumomediastinum | Pneumopericardium | Pneumothorax | Subcutaneous emphysema | |||||
| 1 | 44 | M | + | + | + | + | − | + | + | + | − | + | − | + | − | 1 | Discharged |
| 2b | 67 | M | + | + | + | + | − | + | + | + | − | + | + | + | − | 2 | Died |
| 3 | 48 | M | + | + | + | + | − | + | + | + | + | + | + | − | − | 11 | Discharged |
| 4 | 36 | M | N | N | N | N | N | N | N | N | N | + | + | − | − | 18 | Discharged |
| 5 | 58 | M | + | + | + | + | + | + | + | + | − | − | + | + | + | 14 | Discharged |
| 6 | 46 | M | N | N | N | N | N | N | N | N | N | + | + | − | + | 1 | Died |
| 7 | 75 | F | + | + | + | + | − | + | + | + | − | + | + | − | + | 5 | Died |
| 8 | 62 | M | + | + | + | + | − | + | + | + | − | + | + | − | − | 11 | Discharged |
| 9 | 58 | M | + | + | + | + | − | + | + | + | − | + | + | − | − | 4 | Discharged |
| 10d | 72 | M | N | N | N | N | N | N | N | N | N | + | +a | + | + | 7 | Died |
| 11 | 57 | F | + | + | + | + | − | + | + | + | − | + | + | − | − | 17 | Died |
| 12c | 50 | M | + | + | + | + | − | + | + | + | − | + | + | + | + | 2 | Died |
N: No data available.
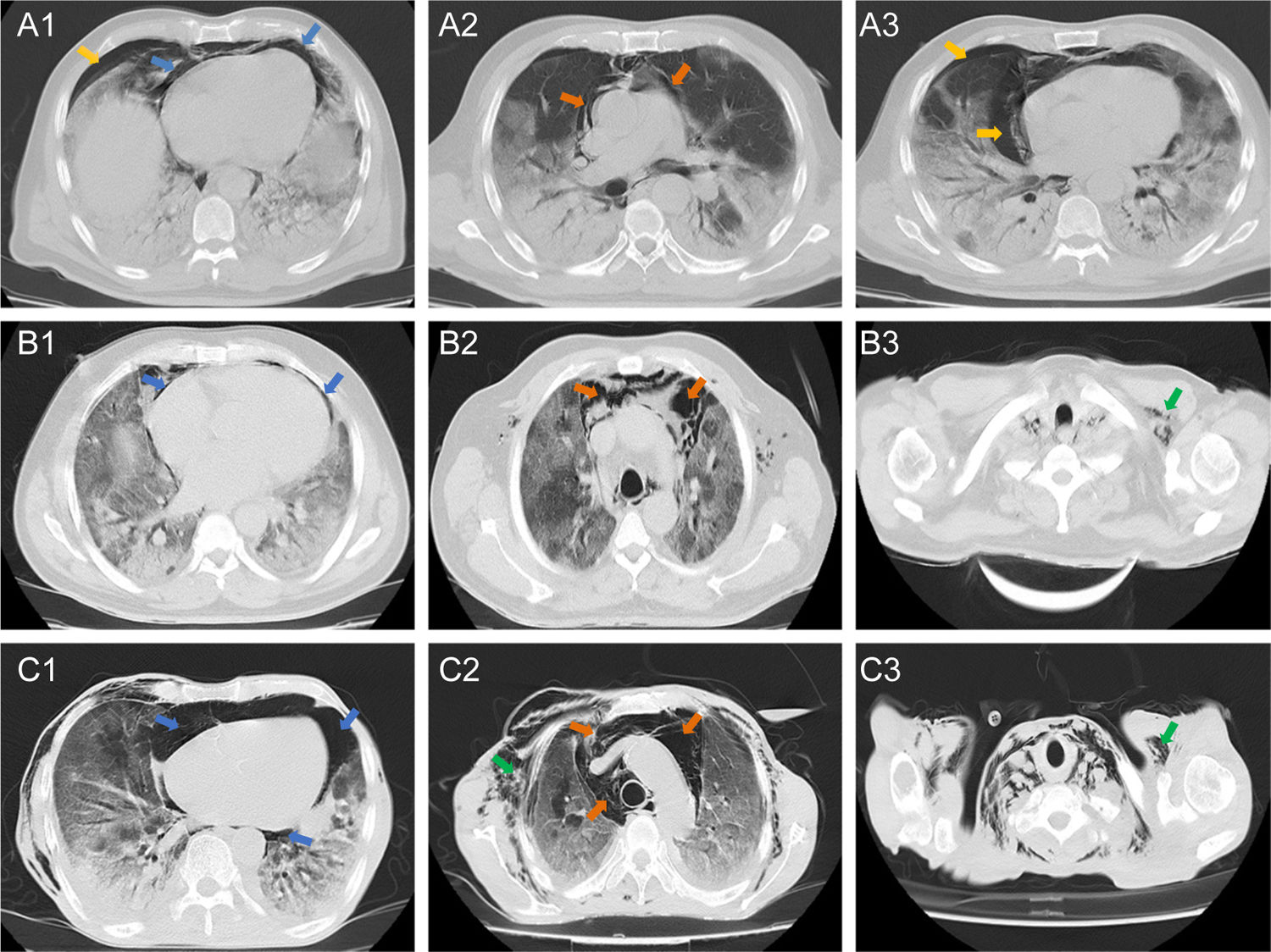
Chest CT showed that ten (83%) patients had both pneumomediastinum and pneumopericardium, while the other two patients had isolated pneumomediastinum or pneumopericardium. Out of the eleven patients with pneumopericardium, one was classified as severe. Pneumothorax was observed in five (42%) patients, four of them accompanied by pneumomediastinum. Pulmonary patterns such as ground glass opacities, crazy paving and consolidation were observed in 100%, 75% and 50% of patients, respectively. Five (42%) patients had subcutaneous emphysema and one of them had subcutaneous neck emphysema. Radiologic findings are presented in Fig. 1.
Axial tomographic sections of three patients (A, B, and C) with different degrees of gas present at the pericardial level (blue arrows), mediastinal (orange arrows), pleural (yellow arrows) and subcutaneous emphysema (green arrows). A1–3 shows a moderate presence of gas around the cardiac silhouette, large vessels, and right pneumothorax. B1 shows presence of gas in the pericardial sac (mild or laminar). C1–3 shows the severe presence of gas in the pericardium, mediastinum and subcutaneous space.
During hospitalization, two patients developed probable pulmonary embolism, one patient developed pericarditis with pericardial effusion and three patients developed acute coronary syndrome. Electrocardiography showed Non-ST elevation; troponin levels were not available. Patients received treatment with anticoagulants, corticosteroids and antibiotics. Additionally, one patient was treated with a single dose of Tocilizumab. All patients required oxygen support and one patient was admitted to the Intensive Care Unit (ICU) for posterior mechanical ventilation. Management was conservative. Six patients (50%) died during hospitalization and the rest were discharged.
Pneumopericardium and pneumomediastinum are the accumulation of air in the pericardial and mediastinal spaces, respectively. They involve the dissection of extrapulmonary air through the thoracic cavity. After alveolar rupture, air enters interstitial tissue spaces where it can remain as primary interstitial emphysema or continue dissecting along the perivascular and peribronchial connective tissue sheaths into the midline. The pressure gradient is responsible for directing the dissection along vascular sheaths toward the hilum. Afterwards, the air can assemble in the mediastinum to form a pneumomediastinum, rupture the pleura to form a pneumothorax or enter the pericardial space between the parietal and visceral layers.12 This could explain why most of our patients had both pneumopericardium and pneumomediastinum and why 42% of our patients presented with pneumothorax.
The most frequent causes of pneumopericardium are secondary to trauma, positive pressure through mechanical ventilation, or invasive chest procedures. Pneumomediastinum most commonly occurs by mechanical ventilation or airway obstruction due to an increase in airway pressure. It is unusual to observe cases of these events as complications of respiratory infections, particularly those with a viral etiology.13
During the SARS outbreak in 2002, a retrospective study14 of 123 patients with SARS-CoV infection reported 13 cases of spontaneous pneumomediastinum. Five of these patients required posterior intubation and four patients died. The probability of encountering these conditions increases if the viral infection leads to SARS and if the patient requires mechanical ventilation or central line placement. In COVID-19, all mechanisms of lung damage (direct injury, exaggerated inflammatory response and hypercoagulability),15 could increase the risk of pneumopericardium, pneumomediastinum, and the possibility of positive pressure injury due to mechanical ventilation. Furthermore, although most forms of pneumomediastinum and pneumopericardium are self-limiting, they can sometimes be fatal and require invasive interventions.12
During a hyperinflammatory process such as the one observed in severe cases of COVID-19, alveolar rupture is a possible outcome. Because visceral layers of the deep cervical fascia are contiguous with the mediastinum, air from a pneumomediastinum may decompress into the neck, avoiding a pneumothorax and a physiologic tamponade. It would require an elevated pressure in the deep cervical fascia to redirect air toward other planes which could explain why encountering a pneumopericardium is so infrequent. Nevertheless, in the context of elevated pressures that exceed containment, because of continuity, spontaneous pneumopericardium can still be observed, most frequently, in the presence of pneumomediastinum. Despite pericardial tightness, support of the pericardial reflections is weak at the venous sheaths and could present as a gateway for air.12 Ultimately, it is rare to encounter isolated spontaneous pneumopericardium (not fully understood) and more common to find spontaneous isolated pneumomediastinum or, as our findings show, in conjunction with pneumothorax and subcutaneous emphysema.
Patients with pneumopericardium and pneumomediastinum can present chest pain exacerbated by deep breath and coughing, dyspnea, neck and throat pain, or dysphonia. In the same line, 50% of our patients developed thoracic pain. Similar to previous reports, all patients required oxygen support and some patients developed other cardiovascular complications. Only one patient presented severe pneumopericardium and consequently died. Finally, we had a higher mortality (50%) compared to the other studies (43%).
Our study has several limitations. We did not have a confirmatory test for COVID-19 and a subgroup of patients was diagnosed only by symptomatic and radiologic findings due to limited resources. We obtained a small sample size and clinical records of three patients were not found. Finally, it was not possible to calculate the incidence of these complications.
FundingThis research has not received any funding.
Conflict of interestThe author(s) declare(s) no conflict of interest.