We would like to commend Gutierrez-Morales et al. on their interesting case presentation of spontaneous pneumomediastinum in an asthmatic patient published in your esteemed journal.1 We wish to extend the spectrum of this rare phenomenon presenting with perplexing clinical and radiological findings which make the management and decision making a challenging task.
A 62-year-old man in the medical ICU was being treated for alleged asthma exacerbation. He has had no past admissions for asthma exacerbation. On clinical examination his blood pressure was 114/86mm HG with heart rate of 96 beats per minute. He was saturating 97% on 50% FiO2 on bi-level non-invasive ventilation for his work of breathing. Chest auscultation revealed prolonged expiratory phase with shortness of breath worse in supine position. There was equal but diminished air entry on both sides with equal chest wall movement. There was no subcutaneous crepitus on palpitation. During treatment with non-invasive ventilation patient developed subcutaneous emphysema (extensive subcutaneous crepitus) and progressive shortness of breath requiring intubation and mechanical ventilation. Extensive subcutaneous crepitus was noted on palpation as well as on auscultation of the chest and precordium (Hammond's crunch).
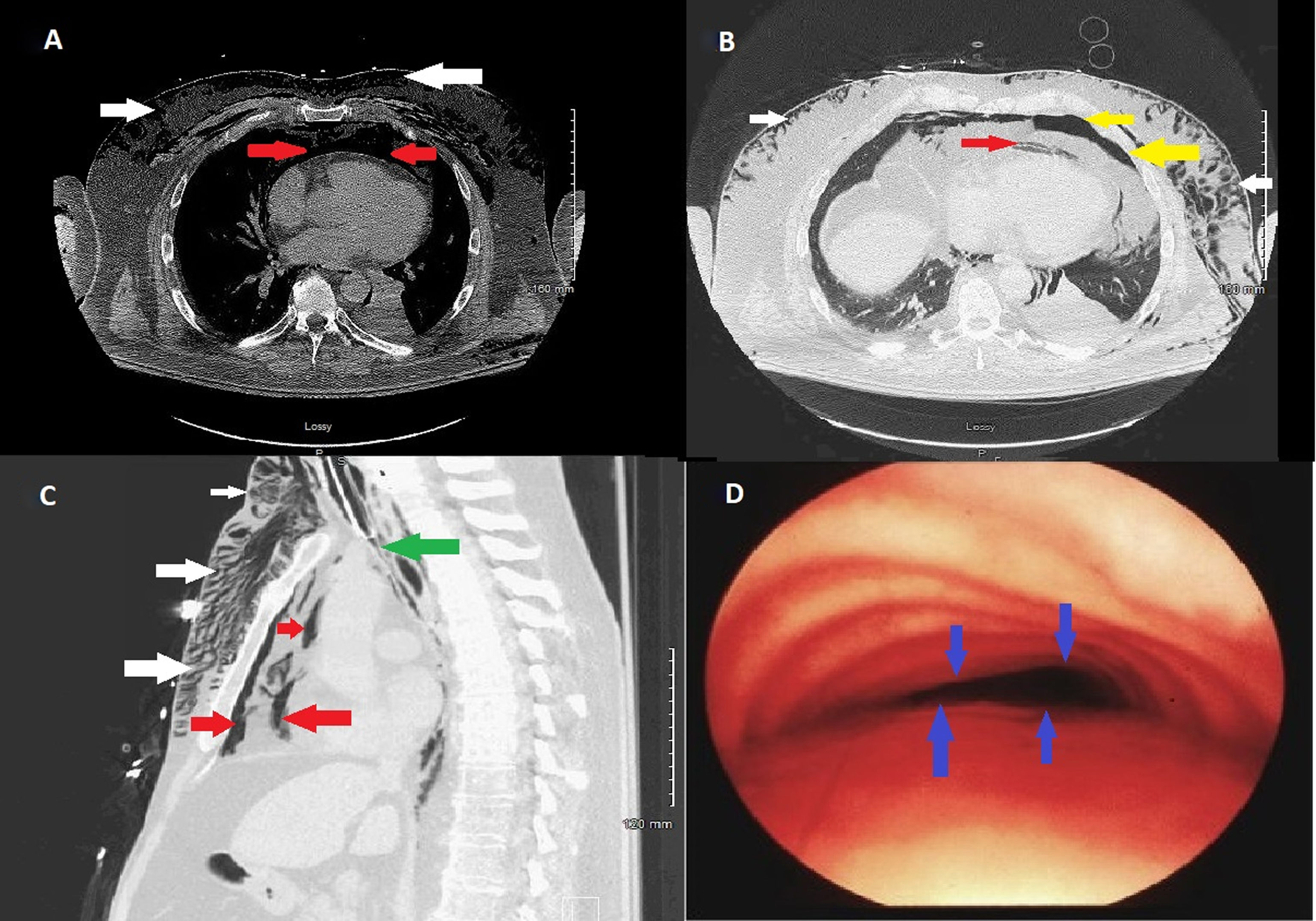
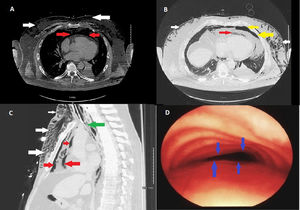
Chest X-ray done at the time showed extensive subcutaneous emphysema. Due to progressive shortness of breath and hypoxia, patient was intubated and pharmacological paralysis was induced to aid effective ventilation. Computed tomography (CT) of the chest showed severe subcutaneous emphysema (Fig. 1A/B/C white arrows), moderate to severe pneumomediastinum (Fig. 1A/B/C red arrows) and moderate sized left basal pneumothorax (Fig. 1B yellow arrows). Interestingly reporting radiologist also noted complete collapse of the trachea distal to the endotracheal tube (ETT) (Fig. 1C green arrow) and suggested tracheal/bronchial rupture as a differential diagnosis in the current clinical context. However, there was no loss of peak or plateau airway pressures on the mechanical ventilator. At this point, patient's oxygen requirements had increased requiring 100% FiO2 for optimal oxygen saturation with hypotension with systolic blood pressure of 90mm Hg. Urgent surgical consultation was sought and patient was anticipated to undergo extra corporeal membrane oxygenation (ECMO) prior to surgical correction of suspected tracheal rupture.
(A) Axial view computed tomography of chest (mediastinal window) showing pneumomediastinum (red arrows) and subcutaneous emphysema (white arrows). (B) Axial view computed tomography of chest (lung window) showing pneumomediastinum (red arrows), subcutaneous emphysema (white arrows) and left basal pneumothorax (yellow arrows). (C) Sagittal view computed tomography of chest (lung window) showing pneumomediastinum (red arrows), subcutaneous emphysema (white arrows) and complete collapse of trachea distal to endotracheal tube (ETT) tip (green arrows). (D) Bronchoscopic view showing complete collapse of anterior tracheal wall (blue arrows) typical of tracheomalacia.
Patient underwent emergency bronchoscopy which showed collapsed trachea distal to the ETT tip classic for tracheomalacia (Fig. 1D blue arrows). Focused bronchoscopic examination until the second generation bronchi bilaterally did not reveal any airway wall rupture.
It was concluded that the patient most likely had tracheomalacia which was probably diagnosed as acute asthma exacerbation. The positive airway pressure ventilation most likely caused barotrauma and leading to pneumothorax, pneumomediastinum and extensive subcutaneous emphysema leading to hypoxic respiratory failure. Our patient subsequently underwent tube thoracostomy for left sided pneumothorax and conservative management for pneumomediastinum. He was extubated 7 days later and made complete recovery.
Spontaneous pneumomediastinum (SPM) is also known as Hamman's syndrome. SPM is a rare entity and is characterized by air leak into the mediastinum, not secondary to any underlying disease.2 It was first described by Macklin as “the transference of air along sheaths of pulmonic blood vessels from alveoli tomediastinum” it can be shortened as follows: alveolar ruptures leading to air dissection along bronchovascular sheaths, and spreading into the mediastinum.3 Hamman's syndrome is not a life-threatening condition and, once diagnosed, may require only supportive and symptomatic therapy, which includes oxygen, analgesics and sedatives as necessary, unless it is associated with tension pneumothorax and/or hemodynamic instability as noted in our patient.4–7 Secondary pneumomediastinum can be differentiated from SPM when a causative factor is identified. It could be iatrogenic (endoscopic procedures, airway manipulation such as during endotracheal intubation, pleural cavity instrumentation, central venous access procedures, blunt or penetrating trauma, inhalation of toxic fumes etc.). Addressing the underlying cause usually should suffice and conservative management would be the choice of treatment. However, in rare cases the simple pneumomediastinum can progress to a malignant one and can lead to hemodynamic instability due to cardiac tamponade and occasionally airway compromise. In these cases video-assisted thoracoscopic surgery becomes an indispensable tool for decompression and attaining hemodynamic stability.
Due to its acute presentation and often other concomitant illnesses, spontaneous pneumomediastinum creates a cause of worry for the treating physicians. Quest for diagnosis and underlying cause is of prime importance, since it has an impact on overall prognosis and management plans. Chest X rays and CT scans are the investigative modalities of choice for a conclusive diagnosis.8-10 Despite better visualization of structures on CT chest, there are instances where the source of free air in the mediastinum has been misdiagnosed. Brussa et al. described a case of spontaneous pneumomediastinum in a pregnant patient, CT scan of the chest was over-read as possible tracheal rupture but bronchoscopy was able to avert the unnecessary surgical exploration.11 Bronchoscopy often provides precious diagnostic information in critically ill patients where the cause of hypoxia is elusive, such as lobar torsion after surgery, mucus plugging and lung collapse.12 Similar mistake occurred in the radiological assessment of our patient which triggered a need for a major surgical intervention and possibly ECMO. In our case too, bronchoscopy was able to provide a more accurate diagnosis and eliminate the doubts of tracheal rupture. It is understandable though, that in a subset of critically ill patients, requiring high fraction of inspired oxygen and positive end expiratory pressure (PEEP), bedside bronchoscopy may not be an available option.
Our case highlights a few important teaching points. Firstly, misdiagnosis of tracheomalacia as asthma exacerbation led to anchoring bias which changed the trajectory of treatment. Secondly, radiological misreading of tracheomalacia as possible tracheal/bronchial rupture almost resulted in unnecessary major surgical intervention. Traumatic intubation can lead to pneumothorax, pneumomediastinum and respiratory failure.13 Accuracy in radiological reading is highly desirable in such cases, however, there have been instances where over-reading may result in possible (unnecessary) surgical intervention.11 Pneumomediastinum is an alarming condition and can be associated with high mortality if infection ensues, despite surgical management. Bronchoscopy should be strongly considered before committing to surgical intervention when tracheal wall rupture is suspected on CT scan to confirm diagnosis and negate erroneous reading.5,11