International and Spanish guidelines recommend a 4-drug regimen in the intensive treatment of tuberculosis (TB). The aim of our study was to determine if these recommendations are followed in Spain, and the factors associated with the use of 3 drugs (standard regimen without ethambutol).
MethodologyObservational, multicenter, retrospective analysis of data from patients diagnosed with TB in practically all Spanish Autonomous Communities between 2007 and 2102. Factors associated with the use of 3 drugs were analyzed using logistic regression, and odds ratios (OR) and corresponding 95% confidence intervals (CI) were calculated.
ResultsA total of 3189 patients were included, 1413 (44.3%) of whom received 3 drugs. The percentage of 3-drug users among patients with positive sputum smear was 41.2%; among patients with resistance to at least 1 drug, 36.1%; among HIV-infected patients, 31.4%; and among immigrants, 24.8%. Factors associated with the use of 3 drugs were: female sex (OR=1.18; CI: 1.00–1.39); native Spanish (OR=3.09; CI: 2.58–3.70); retired (OR=1.42; CI: 1.14–1.77); homeless (OR=3.10; CI: 1.52–6.43); living alone (OR=1.62; CI: 1.11–2.36); living in a family (OR=1.97; CI: 1.48–2.65); seen by specialists in the health area (OR=1.37; CI: 1.10–1.70); no HIV infection (OR=1.63; CI: 1.09–2.48); and negative sputum smear with positive culture (OR=1.59; CI: 1.25–2.02).
ConclusionsA large proportion of TB patients receive intensive treatment with 3 drugs. TB treatment recommendations should be followed, both in routine clinical practice and by the National Plan for Prevention and Control of Tuberculosis in Spain.
Las normativas internacionales y españolas recomiendan el tratamiento intensivo de la tuberculosis (TB) con cuatro fármacos. El objetivo es determinar si en España se sigue esta recomendación y los factores asociados a utilizar tres fármacos (pauta estándar sin etambutol).
MetodologíaEstudio multicéntrico descriptivo, retrospectivo, en el que se analizaron los datos de los pacientes diagnosticados de TB en prácticamente todas las Comunidades Autónomas españolas entre 2007 y 2012. El estudio de factores asociados a prescribir tres fármacos se basó en regresión logística, calculándose las odds ratio (OR) y sus correspondientes intervalos de confianza del 95% (IC).
ResultadosSe incluyeron 3.189 pacientes, de los que 1.413 (44,3%) fueron tratados con tres fármacos. Este porcentaje fue del 41,2% en los pacientes con baciloscopia positiva, del 36,1% en los que tenían al menos resistencia a un fármaco, del 31,4% en los que tenían infección por VIH y del 24,8% en los inmigrantes. Los factores asociados al uso de tres fármacos fueron: ser mujer (OR=1,18; IC: 1,00-1,39); ser autóctono (OR=3,09; IC: 2,58-3,70); estar jubilado (OR=1,42; IC: 1,14-1,77); vivir sin techo (OR=3,10; IC: 1,52-6,43), vivir solo (OR=1,62; IC: 1,11-2,36) o en familia (OR=1,97; IC: 1,48-2,65); ser atendido por especialistas de zona (OR=1,37; IC: 1,10;1,70); no estar infectado por el VIH (OR=1,63; IC: 1,09-2,48) y tener baciloscopia negativa con cultivo positivo (OR=1,59; IC: 1,25-2,02).
ConclusionesExiste una proporción importante de tratamiento intensivo con tres fármacos. Se deben seguir las recomendaciones del tratamiento de la TB, tanto en la práctica clínica habitual como por parte del Plan para la Prevención y Control de la TB en España.
International1–3 and Spanish4–7 guidelines for the treatment of tuberculosis (TB) routinely recommend the standard administration of 4 drugs (isoniazid, rifampicin, pyrazinamide, and ethambutol) in the initial or intensive treatment phase of the disease. However, there is some debate as to whether the administration of the fourth drug (ethambutol) in the intensive phase can be avoided, depending on the level of resistance to antituberculous drugs in that region or the type of TB the patient presents (disease site, microbiological data).
The main reason for including ethambutol in the intensive phase is to initiate treatment with a sufficient number of effective drugs, in order to offset any existing resistance to first line drugs and to avoid the development of new resistances. Hence, the most important consideration when prescribing a 4-drug regimen is the national or regional resistance pattern. Nevertheless, in today's globalized world in which people constantly migrate from country to country, it is difficult to predict if a specific patient will be resistant or not. Aside from widespread population movements, TB has widely varying mechanisms for transmission, meaning that individuals with highly resistant strains can transmit the disease to groups traditionally associated with drug sensitive strains, and vice versa. For this reason, international and Spanish guidelines recommend the systematic use of 4 drugs in the intensive treatment of pulmonary TB, at least until the antibiotic resistance profiles have been established.
A recent national multicenter study in tuberculosis resistance in Spain,8 found 6.7% cases of isoniazid-resistant and 1.9% cases of multi-drug resistant (MDR) TB (resistant to at least hydrazides and rifampicin). Moreover, a preliminary analysis of the use of 3 or 4 drugs in the intensive treatment of TB suggested that a high percentage of cases in Spain were treated with 3 drugs,9 despite the indications given in different guidelines.
In view of this situation, we identified the need for a study to determine if the guideline recommendations for intensive TB treatment with 4 drugs were being followed in Spain, and to identify the factors associated with prescribing only 3 drugs (standard regimen without ethambutol) in the intensive phase.
Material and MethodsSubjectsWe analyzed data from patients with a diagnosis of TB from practically all the Spanish Autonomous Communities (Fig. 1) included in the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) Integrated Programme for Tuberculosis Research (PIITB) database between 2007 and 2012, inclusive. Inclusion criteria were: (a) positive sputum smear, or negative sputum smear and positive culture for Mycobacterium tuberculosis, or in the case of extrapulmonary TB, histological identification of caseating granulomas; (b) patients with clinical, radiological, epidemiological and/or laboratory suspicion leading their physician to recommend the initiation of antituberculous treatment; and (c) informed consent.
DesignThis was a retrospective, descriptive, multicenter study. The information obtained from all patients was stored in electronic case report forms (CRF) accessed by the investigators and study co-workers via a web-based computer platform using a password and user name. Survey and database completion was monitored by phone and email.
Study VariablesAll variables had been collected prospectively in CRFs, as described below: sociodemographic data (age, sex, country of origin, working status, profession, living conditions, data and place of medical attention, smoking history, use of alcohol and other drugs). Associated diseases (HIV, other immunosuppressive conditions). Clinical history (TB history, date of onset of symptoms). Diagnostic methods (date diagnostic tests requested, TB site, associated diseases, sputum smear, culture, radiology, drug sensitivity). Treatment (regimen, treatment start date, planned completion date, clinical progress, treatment adherence, directly observed treatment, understanding of disease and treatment). Final treatment outcome (cure, treatment completed, failure, transfer, dropout, death due to TB, death for other cause, date of death, extended treatment, lost-to-follow-up).
Data AnalysisThe frequency distribution of variables of interest was analyzed, and the proportions between groups were compared on a bivariate level using the χ2 test. Factors associated with the administration of 3 or 4 drugs in the initial treatment were analyzed using the stepwise selection method, including factors that reached significance in the bivariate analysis (P<.05). The odds ratio (OR) and the corresponding 95% confidence intervals (CI) were calculated, and a P value of <.05 was considered significant. The IBM SPSS Statistics 19 software package was used for analysis.
ResultsA total of 3189 patients were included in this study. The population was predominantly male (1940; 60.8%), and 1122 (35.2%) were immigrants. Disease site was the lung in 2476 (77.6%) cases. A total of 1669 (52.3%) had positive sputum smear, 907 (28.4%) had negative sputum smear with positive culture, and 524 (16.4%) had negative sputum smear and negative culture. A total of 2981 (93.5%) had no resistance to any drug, and 208 (6.5%) had resistance to at least 1 drug. A total of 1776 (55.7%) were treated with 4 drugs and 1413 (44.3%) received 3 (Table 1).
Differential Characteristics of the Use of 3 or 4 Drugs in the Initial Phase of Tuberculosis Treatment.
| Total | 4 Drugs | 3 Drugs | |||
|---|---|---|---|---|---|
| n=3189 | n=1776 | % | n=1413 | % | |
| Sex | |||||
| Men | 1940 | 1094 | 56.4 | 846 | 43.6 |
| Women | 1128 | 605 | 53.6 | 523 | 46.4 |
| Country of origin | |||||
| Others | 1122 | 844 | 75.2 | 278 | 24.8 |
| Spain | 2067 | 932 | 45.1 | 1135 | 54.9 |
| Working status | |||||
| Active | 1601 | 953 | 59.5 | 648 | 40.5 |
| Unemployed | 647 | 369 | 57 | 278 | 43 |
| Retired | 524 | 205 | 39.1 | 319 | 60.9 |
| Incapacitated | 40 | 22 | 55 | 18 | 45 |
| Living conditions | |||||
| Group | 372 | 293 | 78.8 | 79 | 21.2 |
| Family | 2269 | 1177 | 51.9 | 1092 | 48.1 |
| Alone | 276 | 158 | 57.2 | 118 | 42.8 |
| Homeless | 42 | 19 | 45.2 | 23 | 54.8 |
| Imprisoned | 38 | 21 | 55.3 | 17 | 44.7 |
| Place of medical attention | |||||
| Emergency room | 1569 | 924 | 58.9 | 645 | 41.1 |
| Specialist unit | 539 | 249 | 46.2 | 290 | 53.8 |
| General practitioner | 616 | 324 | 52.6 | 292 | 47.4 |
| HIV | |||||
| Yes | 137 | 94 | 68.6 | 43 | 31.4 |
| No | 2465 | 1416 | 57.4 | 1049 | 42.6 |
| Microbiology | |||||
| Culture(−) | 524 | 320 | 61.1 | 204 | 38.9 |
| SS(+) | 1669 | 982 | 58.8 | 687 | 41.2 |
| SS(−)/culture(+) | 907 | 421 | 46.4 | 486 | 53.6 |
| Drug resistance | |||||
| Yes | 208 | 133 | 63.9 | 75 | 36.1 |
| No | 2981 | 1643 | 55.1 | 1338 | 44.9 |
| Understanding of the disease and treatment | |||||
| Difficult | 192 | 115 | 59.9 | 77 | 40.1 |
| Easy | 2579 | 1407 | 54.6 | 1172 | 45.4 |
| Year of diagnosis | |||||
| 2009 | 528 | 366 | 69.3 | 162 | 30.7 |
| 2007 | 717 | 373 | 52 | 344 | 48 |
| 2008 | 501 | 301 | 60.1 | 200 | 39.9 |
| 2010 | 443 | 236 | 53.3 | 207 | 46.7 |
| 2011 | 563 | 307 | 54.5 | 256 | 45.5 |
| 2012 | 437 | 193 | 44.2 | 244 | 55.8 |
SS: sputum smear.
Some values do not add up to the total number of cases, since some variables were missing.
The results of the statistical analysis of the characteristics of the cases treated with 3 or 4 drugs and the factors associated with the use of only 3 drugs are shown in Tables 1 and 2, respectively. It is interesting to note that the 4-drug regimen was administered to 982 (58.8%) patients with a positive sputum smear, to 844 (75.2%) immigrants, to 94 (68.6%) HIV-infected individuals, to 293 (77.8%) subjects who lived in a group, and to 133 (63.9%) patients with resistance to at least 1 drug. The 3-drug combination was administered to 687 (41.2%) patients who had a positive sputum smear, 278 (24.8%) immigrant patients, 43 (31.4%) HIV-infected individuals, and 75 (36.1%) patients who were resistant to at least 1 drug.
Factors Associated With the use of Only 3 Drugs in a Cohort of 3189 Patients With Tuberculosis. Univariate Analysis.
| Univariate Analysis | ||
|---|---|---|
| OR (95% CI) | P | |
| Sex | ||
| Men | Ref. | Ref. |
| Women | 1.12 [0.96–1.30] | .139 |
| Country of origin | ||
| Others | Ref. | Ref. |
| Spain | 3.69 [3.15–4.34] | <.001 |
| Working status | ||
| Active | Ref. | Ref. |
| Unemployed | 1.11 [0.92–1.33] | .278 |
| Retired | 2.29 [1.87–2.80] | <.001 |
| Incapacitated | 1.21 [0.63–2.27] | .566 |
| Living conditions | ||
| Group | Ref. | Ref. |
| Family | 3.43 [2.66–4.49] | <.001 |
| Alone | 2.76 [1.96–3.91] | <.001 |
| Homeless | 4.46 [2.31–8.72] | <.001 |
| Imprisoned | 3.00 [1.49–5.97] | .002 |
| Place of medical attention | ||
| Emergency room | Ref. | Ref. |
| Specialist unit | 1.67 [1.37–2.03] | <.001 |
| General practitioner | 1.29 [1.07–1.56] | .008 |
| HIV | ||
| Yes | Ref. | Ref. |
| No | 1.62 [1.12–2.36] | .009 |
| Microbiology | ||
| Culture(−) | Ref. | Ref. |
| SS(+) | 1.10 [0.90–1.34] | .365 |
| SS(−)/culture(+) | 1.81 [1.45–2.25] | <.001 |
| Drug resistance | ||
| Yes | Ref. | Ref. |
| No | 1.44 [1.08–1.94] | .013 |
| Year of diagnosis | ||
| 2009 | Ref. | Ref. |
| 2007 | 2.08 [1.65–2.64] | <.001 |
| 2008 | 1.50 [1.16–1.94] | .002 |
| 2010 | 1.98 [1.52–2.58] | <.001 |
| 2011 | 1.88 [1.47–2.42] | <.001 |
| 2012 | 2.85 [2.19–3.72] | <.001 |
SS: sputum smear.
Ref. is the variable value used as reference in the analysis.
The multivariate analysis confirmed that administration of the 3-drug combination was associated with being female; non-immigrant; retired; homeless, living alone or in a family (using living in a group as reference); having been treated in specialist units (using treatment in the emergency room as reference); no HIV infection; and negative sputum smear and positive culture (Table 3).
Factors Associated With the use of Only 3 Drugs in a Cohort of 3189 Patients with Tuberculosis. Multivariate Analysis.
| Multivariate Analysis | ||
|---|---|---|
| OR (95% CI) | P | |
| Sex | ||
| Men | Ref. | Ref. |
| Women | 1.18 [1.00–1.39] | .039 |
| Country of origin | ||
| Others | Ref. | Ref. |
| Spain | 3.09 [2.58–3.70] | <.001 |
| Working status | ||
| Active | Ref. | Ref. |
| Unemployed | 1.18 [0.96–1.45] | .103 |
| Retired | 1.42 [1.14–1.77] | .001 |
| Incapacitated | 0.80 [0.40–1.58] | .531 |
| Living conditions | ||
| Group | Ref. | Ref. |
| Family | 1.97 [1.48–2.65] | <.001 |
| Alone | 1.62 [1.11–2.36] | .011 |
| Homeless | 3.10 [1.52–6.43] | .001 |
| Imprisoned | 2.03 [0.95–4.27] | .062 |
| Place of medical attention | ||
| Emergency room | Ref. | Ref. |
| Specialist unit | 1.37 [1.10–1.70] | <.001 |
| General practitioner | 1.21 [0.98–1.48] | .062 |
| HIV | ||
| Yes | Ref. | Ref. |
| No | 1.63 [1.09–2.48] | .017 |
| Microbiology | ||
| Culture(−) | Ref. | Ref. |
| SS(+) | 1.00 [0.80–1.25] | .964 |
| SS(−)/culture(+) | 1.59 [1.25–2.02] | <.001 |
| Drug resistance | ||
| Yes | Ref. | Ref. |
| No | 1.30 [0.94–1.79] | .105 |
| Year of diagnosis | ||
| 2009 | Ref. | Ref. |
| 2007 | 2.49 [1.92–3.23] | <.001 |
| 2008 | 1.70 [1.29–2.25] | <.001 |
| 2010 | 2.23 [1.69–2.97] | <.001 |
| 2011 | 2.01 [1.54–2.63] | <.001 |
| 2012 | 2.98 [2.25–3.96] | <.001 |
SS: sputum smear.
Ref. is the variable value used as reference in the analysis.
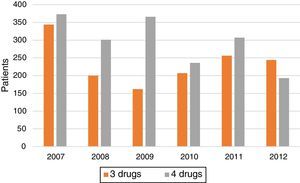
Treatment trends (3 drugs vs 4 drugs) over the years (Fig. 2 and Table 1) show a marked increase in the proportion of cases treated with 4 drugs in 2008 and 2009. After that, rates fall steadily, and by 2012 the proportion of patients receiving 3 drugs was even higher that those receiving 4.
No significant differences were observed in the clinical progress or final treatment outcome between patients receiving 3 or 4 drugs. Treatment was considered satisfactory (cured or treatment completed) in 79.9% of patients who received 3 drugs and in 76.2% of those who received 4. Treatment outcome was satisfactory in 73% of patients who had at least 1 resistance and received 3 drugs, and in 81% of patients with resistances who received 4 drugs. The difference was not significant (P=.18).
Finally, more patients in the drug-resistant group received prolonged treatment than in the group of patients with sensitive strains (13% vs 3.1%; P<.001).
DiscussionThis study in TB patients from practically all the Autonomous Communities of Spain showed that a significant proportion of subjects, some of whom are at risk of developing resistance, were not treated in accordance with the Spanish and international guidelines for intensive 4-drug therapy.
A total of 58% of patients with positive sputum smear received 4 drugs. However, the remaining TB carriers were in a precarious situation, since they could well have had resistant strains that would not be determined until weeks later, at which stage the treatment would have to be changed. Moreover, new resistances may even have been activated and/or transmitted, with the risk of generating cases of MDR TB and extremely drug resistant (XDR) TB (defined as MDR TB associated with resistance to quinolones and at least 1 second-line injectable). This risk may be minimized, at least in part, by the generalized use of new molecular techniques for diagnosing resistance, such as Xpert MTB/RIF assay or GeneXpert®,10 which can detect rifampicin resistance, thus suggesting MDR TB, at the time of diagnosis and start of treatment, or GenoType MTBDRplus11 for the rapid detection of rifampicin and hydrazide resistance.
More patients with negative sputum smear (53.6%) received 3 drugs rather than 4 drugs. The reason for this may be purely practical: after receiving a positive result from microbiology, the treating clinician knows that the lab will shortly send the resistance profile. Therefore, if the patient does have a resistant strain, there will be insufficient time to develop new resistances due to inappropriate treatment.
It is interesting to note that in our study, only 75.2% of the immigrant population received the 4-drug regimen, leaving a quarter of them to receive 3 drugs. This practice is clinically inacceptable, since the resistance rates in immigrant patients are known to be higher than in Spanish nationals (11.9% isoniazid resistance in immigrants, compared to 4.1% in nationals, according to a recent Spanish study).8 Moreover, the percentage of foreign patients among the overall population of TB patients in Spain is 29.7%, according to a previous study,12 and 35% according to our study, underlining the inadvisability and high risk of initiating 3-drug treatment.
Spanish nationals received 3 drugs (54.9%) more often than 4, which may be a reflection of the physicians’ confidence in reports of low resistance rates in this country. However, recent data published by our group8 reveal a higher than expected rate of resistance in Spanish nationals (4.1% isoniazid resistance), suggesting that it is advisable to treat patients with 4 drugs right from the start.
Interestingly, 75 patients with resistance to first-line drugs (36.1% of the total) began treatment with 3 drugs. This may cause significant therapeutic problems for the afore-mentioned reasons and the treatment regimen may need to be amended if the resistance profile results are positive. This further highlights the need to start treatment with 4 drugs, since any patient can present drug-resistance that hampers treatment and can even extend the contagion period by delaying the effect of the treatment. The similar percentage of satisfactory treatment outcomes in patients with sensitive or resistant strains receiving 3 or 4 drugs may be explained by the fact that the Spanish TB physicians who participated in this study are specialists with extensive experience in managing this disease. Therefore, it is reasonable to assume that if resistances had developed, the physicians would have prescribed a new treatment regimen in accordance with the developing resistance, thus achieving a good final outcome for their patients. This is another reason why treatment was extended in more resistant cases (13%) than in cases with sensitive strains (3.1%).
Trends in the use of the 3- or 4-drug regimen over the years of the study show an increase in the use of 4 drugs in 2008 and 2009. This may have been due to the influence of the recent (at that time) publication of the Spanish guidelines. However, rates have subsequently diminished, and by 2012 the proportion of patients receiving 3 drugs was even higher that those receiving 4, showing a growing trend towards ignoring TB guidelines. These data are worrying when viewed alongside the recent increase (according to the latest 2 national multicenter studies)8,13 in both initial isoniazid resistance and MDR TB. Moreover, no specific studies have been performed in the Spanish Autonomous Communities which would justify the administration of 3- or 4-drug therapies on the basis of local resistance and MDR figures. The introduction of Xpert MTB/RIF and other nucleic acid amplification techniques, mentioned above, may change TB morbidity and mortality by improving diagnosis through early detection of resistance. Prompt treatment of new cases will have a significant impact on the dynamics of long-term transmission.11
Very few international studies have evaluated compliance with treatment recommendations in the intensive phase of TB. A Malaysian study reported that only 49.4% of patients completed the 2-month intensive phase, despite the recommendations of the World Health Organization (WHO).14 In France, a significant number of doctors (12.2%) responding to a national questionnaire reported that they systematically used a 3-drug regimen in the initial treatment, instead of the recommended 4 drugs, but the proportion of patients receiving the 3-drug regimen could not be retrieved from the data, since each doctor treated a different number of patients. The authors concluded that efforts needed to be made to achieve compliance with treatment guidelines.15 In India, the Revised National Tuberculosis Control Programme uses an initial regimen, consisting of rifampicin administered intermittently 3 times a week, including HIV-infected patients, while the WHO recommends daily treatment at least in the intensive disease phase: the mortality rate in these patients was high.16
One of the limitations of our study is that we could not show if the use of 3 drugs generated cases of resistance, since no clinical–epidemiological or molecular disease transmission studies were performed. Nor could we demonstrate a difference in the final classification or outcome of patients treated with 3 or 4 drugs, possibly because the level of resistance in Spain is low, and because treatment was administered by physicians with expertise in the treatment of TB: if resistance was determined in the initial tests, they may have decided to switch to an effective regimen. The strength of the study is that it reflects the standard practice of a large group of TB specialists representing practically all the Spanish Autonomous Communities.
ConclusionsDespite current TB treatment guidelines, a significant proportion of patients treated in the intensive phase receive 3 drugs (44.3%). It seems highly likely that a significant proportion of immigrants or non-Spanish natives, patients with positive sputum smears, and patients with initial resistance may have initially received the 3-drug regimen, exposing them to the risk of developing resistance. It is worrying to note that in the last year of the study, more patients received 3 drugs than 4.
For these reasons, we recommend raising awareness of the guidelines, and call on the Plan for Prevention of Control of Tuberculosis in Spain to put in place systems for monitoring compliance with the recommendations of national and international TB treatment guidelines. Four drugs must be prescribed in the intensive phase of the disease, unless results are available from rapid resistance tests in a matter of hours.
AuthorshipJMGG, TR, JAC: study conception. JMGG, TR, JAC: drafting the manuscript. MC: statistical analysis. JMGG, TR, JC, MC: analysis of results. JMGG, TR, MC, JAC, TPP, JRM: critical reading, review and final approval of the manuscript. PIITB Working Group: data collection and final approval of the manuscript.
Conflict of InterestsThe authors declare that they have no conflict of interests.
R. Agüero (Hospital Marqués de Valdecilla, Santander); J.L. Alcázar (Instituto Nacional de Silicosis, Oviedo); N. Altet (Unidad de Prevención y Control de la Tuberculosis, Barcelona); L. Altube (Hospital Galdakao, Galdakao); F. Álvarez Navascués (Hospital San Agustín, Avilés, Asturias); L. Anibarro (Unidad de Tuberculosis de Pontevedra, Vigo); M. Barrón (Hospital San Millán-San Pedro, Logroño); P. Bermúdez (Hospital Universitario Carlos Haya, Málaga), R. Blanquer (Hospital Dr. Peset, Valencia); L. Borderías (Hospital San Jorge, Huesca); A. Bustamante (Hospital Sierrallana, Torrelavega); J.L. Calpe (Hospital La Marina Baixa, Villajoyosa); J.A. Caminero (Complejo Hospitalario Dr. Negrín, Las Palmas de Gran Canaria); F. Cañas (Hospital Insular de Gran Canaria, Las Palmas de Gran Canaria); F. Casas (Hospital Clínico San Cecilio, Granada), X. Casas (Hospital de Sant Boi, Sant Boi de Llobregat), E. Cases (Hospital Universitario La Fe, Valencia); R. Castrodeza (Hospital El Bierzo Ponferrada-León, Ponferrada); J.J. Cebrián (Hospital Costa del Sol, Marbella); J. E. Ciruelos (Hospital de Cruces, Guetxo); A.E. Delgado (Hospital Santa Ana, Motril), M.L. de Souza (Unidad de Prevención y Control de la Tuberculosis, Barcelona); D. Díaz (Complejo Hospitalario Juan Canalejo, La Coruña); B. Fernández (Hospital de Navarra, Pamplona); A. Fernández (Hospital Río Carrión, Palencia); J. Gallardo (Hospital Universitario de Guadalajara, Guadalajara); M. Gallego (Corporación Sanitaria Parc Taulí, Sabadell); M.M. García Clemente (Hospital Central de Asturias, Oviedo), C. García (Hospital General Isla Fuerteventura, Puerto del Rosario); F.J. García (Hospital Universitario de la Princesa, Madrid); F.J. Garros (Hospital Santa Marina, Bilbao), J.A. Gullón (Hospital San Agustín, Avilés, Asturias); C. Hidalgo (Hospital Universitario Virgen de las Nieves, Granada), M. Iglesias (Hospital Marqués de Valdecilla, Santander); G. Jiménez (Hospital de Jaén), M.A. Jiménez (Unidad de Prevención y Control de la Tuberculosis, Barcelona); J.M. Kindelan (Hospital Universitario Reina Sofía, Córdoba); J. Laparra (Hospital Donostia-San Sebastián, San Sebastian); R. Lera (Hospital Dr. Peset, Valencia), T. Lloret (Hospital General Universitario de Valencia, Valencia); M. Marín (Hospital General de Castellón, Castellón); J.T. Martínez (Hospital Mutua de Terrassa, Terrassa); E. Martínez (Hospital de Sagunto, Sagunto); A. Martínez (Hospital de La Marina Baixa, Villajoyosa); J.F. Medina (Hospital Universitario Virgen del Rocío, Seville); C. Melero (Hospital 12 de Octubre, Madrid); C. Milà (Unidad de Prevención y Control de la Tuberculosis, Barcelona); I. Mir (Hospital Son Llàtzer, Palma de Mallorca); C. Morales (Hospital Universitario Virgen de las Nieves, Granada), M.A. Morales (Hospital Cruz Roja Inglesa, Ceuta); V. Moreno (Hospital Carlos III, Madrid); A. Muñoz (Hospital Universitario Carlos Haya, Málaga), L. Muñoz (Hospital Reina Sofía, Cordoba); C. Muñoz (Hospital Clínico Universitario de Valencia, Valencia); J.A. Muñoz (Hospital Universitario Central, Oviedo); I. Parra (Hospital Universitario Virgen de la Arrixaca, El Palmar); A. Penas (Complejo Hospitalario Xeral-Calde, Lugo); J.A. Pérez (Hospital Arnau de Vilanova, Valencia); P. Rivas (Hospital Virgen Blanca, León); J. Rodríguez (Hospital Universitario Virgen de las Nieves, Granada), J. Ruiz-Manzano (Hospital Universitario Germans Trías i Pujol, Badalona); J. Sala (Hospital Universitario Joan XXIII, Tarragona); M. Sánchez (Unidad de Tuberculosis Distrito Poniente, Almería); P. Sánchez (Hospital del Mar, Barcelona); F. Sanz (Hospital General Universitario de Valencia, Valencia); M. Somoza (Consorcio Sanitario de Tarrasa, Barcelona), E. Trujillo (Complejo Hospitalario de Ávila, Ávila); E. Valencia (Hospital Carlos III, Madrid); A. Vargas (Hospital Universitario Puerto Real, Cádiz); I. Vidal (Complejo Hospitalario Juan Canalejo, Corunna); R. Vidal (Hospital Vall d’Hebron, Barcelona); M.A. Villanueva (Hospital San Agustín, Avilés, Asturias); A. Villar (Hospital Vall d’Hebron, Barcelona); M. Vizcaya (Complejo Hospitalario Universitario de Albacete, Albacete); M. Zabaleta (Hospital de Laredo, Laredo); G. Zubillaga (Hospital Donostia-San Sebastián, San Sebastian).
Members of the Working Group of the Integrated Research Program Tuberculosis (PIITB) are presented in Appendix A.
Please cite this article as: García-García J-M, Rodrigo T, Casals M, Ruiz-Manzano J, Pascual-Pascual T, Caylà JA, et al. Cumplimiento en España de la norma de prescribir cuatro fármacos en la fase intensiva del tratamiento estándar de la tuberculosis. Arch Bronconeumol. 2016;52:262–268.