Both short and long sleep duration have been associated with an increased prevalence and incidence of cancer,1,2 but its relationship with cancer aggressiveness remains unknown. We have previously described an association between cutaneous melanoma and sleep-disordered breathing based on a prospective multicentric cohort.3 In the present paper, we describe the results of a post-hoc analysis aiming to evaluate the association between subjective sleep duration and objective parameters of melanoma aggressiveness.
This is an observational, cross-sectional, multicenter study that included 443 consecutive patients with a diagnosis of melanoma from 29 Spanish hospitals. Patients were excluded if they had in situ melanoma or had received previous treatment with continuous positive airway pressure. The study was approved by the ethics committees of all the hospitals, and all the patients gave their informed consent. Each patient completed a clinical questionnaire which included anthropometric measurements, relevant antecedents, medication, sleep apnea symptoms, presence of insomnia and somnolence (Epworth Sleepiness Scale).
Sleep duration was assessed by asking the patients the following question: How many hours of sleep (including naps) have you had on average in a 24-h period during the last year (year prior to the diagnosis of melanoma)? Participants estimated habitual sleep duration using full hour units. Snoring time was also quantified in the sleep study records.
All patients underwent a sleep study by means of a home respiratory polygraphy and a peripheral blood test. Patients were divided into three groups depending on their daily sleep duration: appropriate sleep duration (between 6 and 8h), short sleepers (<6h) and long sleep duration (>8h). The independent relationship between melanoma aggressiveness factors and sleep duration was determined by introducing into a multivariate logistical regression analysis those variables which could, in the opinion of the researchers, also have clinical importance as confounders: age, sex, AHI, BMI and hypnotics intake. This relationship was evaluated by Hazard Ratio (CI95%), considering the group of patients with appropriate sleep duration as the control group. The degree of melanoma aggressiveness was measured by histological variables such as the Breslow thickness, the tumor mitotic rate (≥5 vs. <5 mitoses per mm2), the histological presence of ulceration and regression and positive sentinel lymph node (SLN) involvement. The cut-off points for the Breslow thickness were established at 1, 2 and 4mm, according to international guidelines.4
443 patients were finally included in the study. Mean age was 55.9±15.3 years, and 50.6% were male. The mean BMI was 27.3±4.5kg/m2 and the median Epworth score was 6 (IQR: 3–8). The median of the Breslow thickness was 0.85mm (IQR: 0.49–1.80). A Breslow thickness above 1, 2 and 4mm was found in, respectively, 44, 22.3 and 8.8% of the patients. Ulceration was present in 16%, regression in 23.3%, and SLN was positive in 10.6% of patients. The median AHI was 8.6 (IQR: 2.8–20.2). The mean sleep duration was 7.4±1.27h, with 4.7% sleeping <6h, 77.9% between 6 and 8h and 17.4%>8h. 8.4% of the patients presented insomnia and 12% were taking hypnotic drugs. The sleep study time was 7.2h (IQR: 6.6–8) and the snoring study time was 6.9h (IQR: 6.5–7.7). The correlation between subjective sleep duration and snoring study time was r=0.86, p<0.0001.
There was a statistically significant correlation between sleep duration and the Breslow index (r: 0.26; p=0.001). Those patients with more aggressive melanoma (Breslow≥2mm vs <2mm, and Breslow≥4mm versus <4mm) presented longer sleep duration (8.1 vs. 7.1h; p=0.0001 and 8.3 vs. 6.9h; p=0.0001, respectively). Long sleepers presented, in comparison with those patients who slept less than 8h, a significant increase in various systemic markers of inflammation like high-sensitivity C-reactive protein (hs CRP) (2.35 [2.3) vs 1.33 [2.5] mg/L; p=0.03), fibrinogen concentration (392 [97.1] vs.335 [92.7] mg/dL; p=0.01) and erythrocyte sedimentation rate (ESR) (17.4 [13.9] vs. 11.2 [9.1] mm1h; p=0.01), as well as a higher AHI (19.8 [17.9] vs. 15.1 [16.1]; p=0.02).
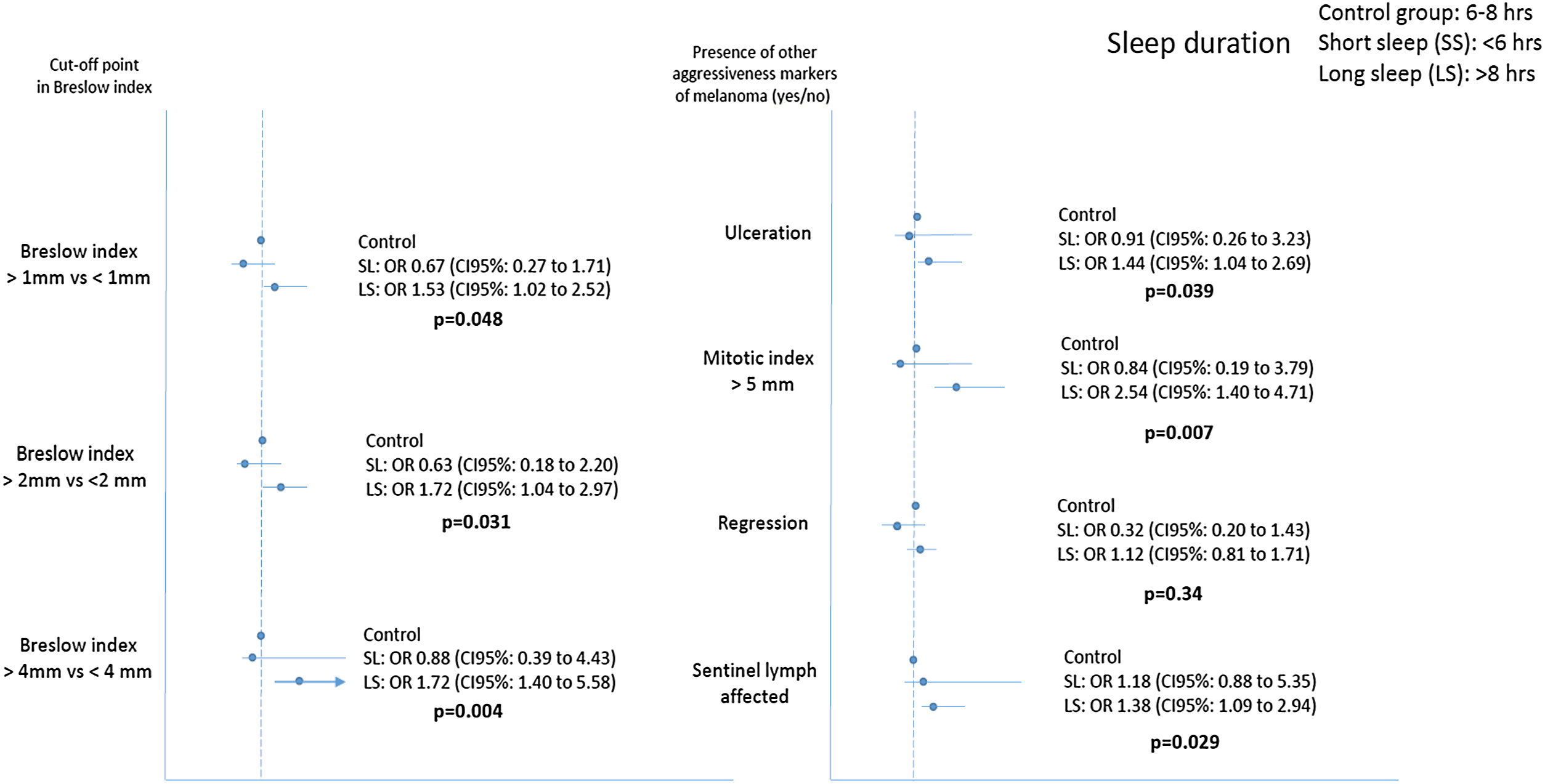
Fig. 1 shows that long sleepers were 1.53, 1.72 and 2.84 times more likely to present a more aggressive melanoma (Breslow index above 1, 2 and 4mm) compared to the control group, after adjustment for age, gender, BMI, AHI, intake of hypnotic drugs and presence of insomnia. Similarly, long sleepers had an increased risk of SLN positive, presence of tumor ulceration and a high tumor mitotic rate.
Relationship between sleep duration and commonly used clinical markers of tumor aggressiveness in cutaneous melanoma. p values refer to sleep duration>8h versus control group (sleep duration between 6 and 8h). Results were adjusted for age, sex, apnea-hypopnea index, body mass index, presence of insomnia and intake of hypnotics. SL: Short sleep; LS: Long Sleep.
As far as we know, this is the first study to analyze the relationship between longer sleep duration and the aggressiveness of a cancer. Of note, this association was not caused by the presence of confounders such as obesity, sleep apnea, age, gender, hypnotics intake or insomnia. Various mechanisms have been postulated to explain this relationship, including various deregulations of the immune system (especially reductions in natural killer cell activity),5 increased sleep fragmentation3 and increased systemic inflammation.6,7 In fact, our study shows that long sleepers presented greater systemic inflammation (increased levels of US-CRP, ESR and fibrinogen).
The main limitation of our study was the fact that the evaluation of sleep duration was subjective, as is the case in most of the similar studies published to date on this topic. However, there was a very strong correlation between the subjective sleep duration and the snoring study time (while the patients are supposed to be sleeping). Moreover, some authors8 have observed in large series that those patients with self-reported sleep>7h per night overestimated their real sleep duration by an average of 0.3h, while this overestimate was even higher (1.3h) in patients who slept<5h. These circumstances would minimize any error derived from our analysis since, even assuming this situation, the vast majority of the patients in our study would remain in the same analysis group. Moreover, the percentage of patients with abnormal sleep time was 22%, which could affect the conclusions. Finally, we did not include the presence of anxiety or depression as a covariate, but we did include the intake of hypnotics and other psychotropic medication, which is closely associated with anxiety and depression.
In conclusion, we found for the first time a positive relationship between a long sleep duration and some markers of melanoma aggressiveness. Future studies are needed to investigate the main pathophysiological mechanisms that could explain this association and the prognostic relevance of this finding.
FundingThe authors have nothing to declare in relation to this manuscript.
Conflict of interestThe authors declare that they have no conflict of interest.