The recent introduction of immunotherapy in the treatment of cancer has revolutionized the management of some tumors, including lung cancer,1 and this treatment is being progressively included in the various therapeutic guidelines. Pembrolizumab is a humanized anti-PD1 monoclonal antibody that is approved in Spain for the treatment of non-small cell lung cancer, in both first and second-line treatment in selected patients,2 and in metastatic melanoma. We report the case of a patient with a diagnosis of metastatic melanoma treated with pembrolizumab, who developed a granulomatous sarcoidosis-like reaction to this treatment, a little-known adverse effect that is rarely described in the literature.
This was a 72-year-old man, former smoker of 50 pack-years, with no respiratory history, diagnosed with a superficial melanoma after excision of a mole from the lower left limb in 2014, with subsequent widening of surgical borders. After 2 disease-free years, metastases were detected in the left inguinal lymph nodes, and lymphadenectomy was performed. Unresectable mesenteric lymphadenopathies subsequently appeared, so treatment began with pembrolizumab every 3 weeks.
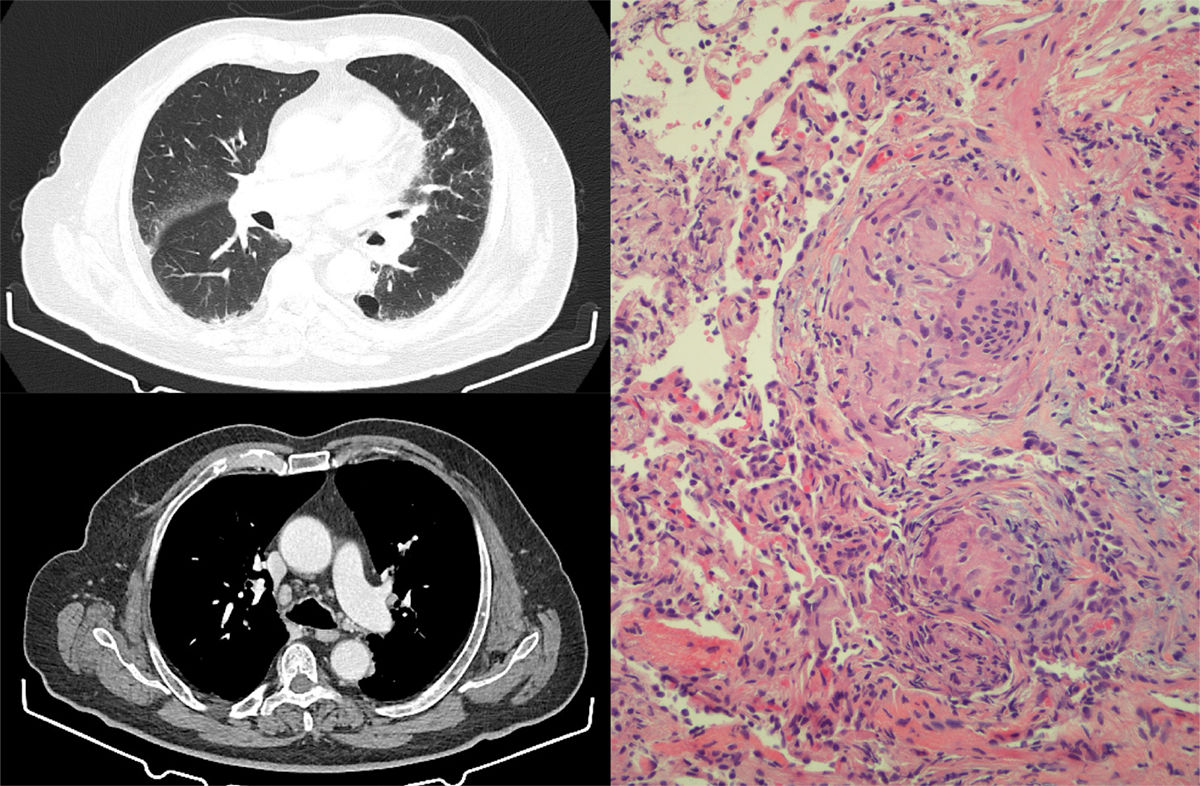
After 6 months of treatment, with partial response of the lymphadenopathies, the respiratory medicine department was consulted due to the appearance on a follow-up chest CT of septal thickening, nodules predominantly in the subpleural and fissure regions, and hilar and mediastinal lymphadenopathies measuring up to 15mm, changes highly suggestive of a sarcoidosis-like granulomatous reaction (Fig. 1). Chest CT before starting treatment showed no changes in the pulmonary parenchyma and no mediastinal lymphadenopathies.
Left: chest HRCT slices showing septal thickening, with nodules predominantly in the subpleural and fissure regions. Mediastinum showing presence of hilar and mediastinal lymphadenopathies measuring up to 15mm. Right: transbronchial biopsy specimen showing non-caseifying epithelioid granulomas. Multinucleated giant cells grouping to form granulomas with scant accompanying lymphocytic cellularity. No necrosis identified.
The patient was asymptomatic at all times, with no respiratory symptoms, cough, dyspnea or fever, nor did he present new skin lesions. The examination revealed bibasal crackles and edema of the lower limbs (secondary to lymphadenectomy), and basal pulse oximetry was 95%. Angiotensin-converting enzyme was 54U/l and functional respiratory tests were normal. Fiberoptic bronchoscopy was performed, and no endobronchial changes were visualized. Bronchoalveolar lavage was consistent with lymphocytic alveolitis, with a CD4/CD8 ratio of 1.1. Five transbronchial biopsies were obtained that showed non-caseifying epithelioid granulomas (Fig. 1). All microbiological analyses of lavage and biopsy material were negative, including mycobacteria testing.
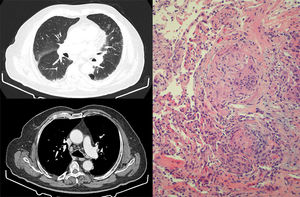
Given the consistent radiological and histological findings and the correlation in time with the treatment, the patient was diagnosed with sarcoidosis-like granulomatous reaction associated with pembrolizumab. As he was asymptomatic and lung function was normal, we decided, in consultation with the oncology department, to discontinue pembrolizumab and to monitor the patient. In the chest CT performed 1 month after withdrawing treatment, partial radiological improvement was seen, both in parenchymal involvement and in the size of the lymphadenopathies, so this approach was maintained. Six months after discontinuing the drug, the follow-up CT showed that the radiological changes had resolved almost completely. The good response to merely discontinuing pembrolizumab confirmed that the sarcoid reaction was directly related to the treatment.
Immunotherapy is defined as the set of treatments aimed at strengthening the immune system to promote the development of antitumor activity. These drugs act by unblocking key inhibitory lymphocyte pathways, such as the CTLA-4 or PD-1/PDL-1 pathway, resulting in the stimulation of T cell activity.3 This activation helps the immune system to attack tumor cells, although it can also increase the chance of host tissue reactions. For this reason, adverse effects associated with these treatments are often autoimmune events, the most common being skin rashes, colitis, liver disease, pneumonitis, and endocrine diseases. The respiratory adverse effects reported in clinical trials include cough, dyspnea, bronchitis, organizing pneumonia, hoarseness, pulmonary fibrosis, and particularly, pneumonitis, which is potentially the most serious.4,5 A meta-analysis of 653 patients treated with pembrolizumab estimated a rate of pneumonitis of 6%, and described 5 typical radiological patterns: cryptogenic organizing pneumonia, ground glass pattern, hypersensitivity type, interstitial, and non-specific.6
Sarcoidosis-like granulomatous reactions have already been described in association with other cancer treatments, such as alpha-interferon or cisplatin,7 or monoclonal antibodies, such as anti-tumor necrosis factor or anti-CD20. These reactions have also been described recently in some cases in association with immunotherapy. In 2008, the first case of sarcoidosis-like granulomatous reaction associated with ipilimumab (anti-CTLA-4) was reported in a patient with metastatic melanoma.8 Another 13 cases with this drug were subsequently published, 12 in the treatment of melanoma and 1 in prostatic adenocarcinoma. Three cases associated with nivolumab (anti-PD1), also in melanoma, have been published, the first in 2016.9 With regard to treatment with pembrolizumab, only 5 confirmed cases have been described, 4 in melanoma10–12 and 1 in metastatic leiomyosarcoma.13 Another 3 cases of reactivation of granulomatous disease prior to initiating immunotherapy (2 treated with pembrolizumab14 and 1 with a combination of ipilimumab and nivolumab) have been published.
These reactions are treated by discontinuing the drug and administering corticosteroids, depending on the degree of involvement. According to guidelines for managing immune-mediated adverse effects,5,15 asymptomatic cases can be managed simply with close monitoring, while corticosteroids should be reserved for the appearance of symptoms or progression, while immunosuppressants can be added in severe cases. In all published cases, sarcoid involvement concluded with resolution of the lesions, irrespective of the use of corticosteroids or drug suspension. The adverse effects of our patient, for example, resolved almost completely merely by discontinuing pembrolizumab.
In conclusion, it is important to remain alert to the possibility of this adverse effect: very few cases have been described to date, but the increase in the use of immunotherapy and the widespread use of these drugs in more and more types of cancer may prompt a significant increase in the incidence of these reactions in coming years. Moreover, we should be careful about assuming that the appearance of mediastinal lymphadenopathies in patients receiving these drugs is due to tumor progression, since it may be a granulomatous reaction, and the diagnosis must be confirmed by biopsy.
Please cite this article as: Gayá García-Manso I, García Ródenas MdM, Barroso Medel ME, Illán Gambín FJ. Reacción granulomatosa sarcoidosis-like asociada al tratamiento con inmunoterapia (pembrolizumab). Arch Bronconeumol. 2018;54:592–593.