The use of noninvasive ventilation (NIV) in non-COPD patients with pneumonia is controversial due to its high rate of failure and the potentially harmful effects when NIV fails. The purpose of the study was to evaluate outcomes of the first ventilatory treatment applied, NIV or invasive mechanical ventilation (MV), and to identify predictors of NIV failure.
MethodsHistorical cohort study of 159 non-COPD patients with pneumonia admitted to the ICU with ventilatory support. Subjects were divided into 2 groups: invasive MV or NIV. Univariate and multivariate analyses with demographic and clinical data were performed. Analysis of mortality was adjusted for the propensity of receiving first-line invasive MV.
ResultsOne hundred and thirteen subjects received first-line invasive MV and 46 received first-line NIV, of which 27 needed intubation. Hospital mortality was 35%, 37% and 56%, respectively, with no significant differences among groups. In the propensity-adjusted analysis (expressed as OR [95% CI]), hospital mortality was associated with age (1.05 [1.02–1.08]), SAPS3 (1.03 [1.00–1.07]), immunosuppression (2.52 [1.02–6.27]) and NIV failure compared to first-line invasive MV (4.3 [1.33–13.94]). Compared with invasive MV, NIV failure delayed intubation (P=.004), and prolonged the length of invasive MV (P=.007) and ICU stay (P=.001). NIV failure was associated with need for vasoactive drugs (OR 7.8 [95% CI, 1.8–33.2], P=.006).
ConclusionsIn non-COPD subjects with pneumonia, first-line NIV was not associated with better outcome compared with first-line invasive MV. NIV failure was associated with longer duration of MV and hospital stay, and with increased hospital mortality. The use of vasoactive drugs predicted NIV failure.
El uso de ventilación no invasiva (VNI) en pacientes sin EPOC con neumonía es motivo de controversia, debido a la elevada tasa de fracasos y a los efectos potencialmente nocivos de dicho fracaso. La finalidad de este estudio fue evaluar la evolución de los pacientes en función de cuál había sido el primer tipo de soporte ventilatorio aplicado, VNI o ventilación mecánica (VM) invasiva, e identificar factores de predicción del fracaso de la VNI.
MétodosEstudio de una cohorte histórica de 159 pacientes sin EPOC con neumonía ingresados en la UCI que recibieron soporte ventilatorio. Los pacientes se clasificaron en dos grupos, VM invasiva y VNI. Se efectuaron análisis univariantes y multivariantes de los datos demográficos y clínicos. El análisis de mortalidad se ajustó por índice de propensión a ser conectado a VM invasiva como tratamiento inicial.
ResultadosCiento trece pacientes fueron conectados a VM invasiva como tratamiento ventilatorio inicial y 46 a VNI, 27 de los cuales precisaron intubación. La mortalidad hospitalaria fue del 35, 37 y 56%, respectivamente, y no se observaron diferencias significativas entre grupos. En el análisis ajustado a la propensión (expresada mediante (OR [IC 95%]), la mortalidad hospitalaria se asoció con la edad (1,05 [1,02 - 1,08]), la puntuación SAPS3 (1,03 [1,00 - 1,07]), la inmunosupresión (2,52 [1,02 - 6,27]) y el fracaso de la VNI, en comparación con la VM invasiva de primera línea (4,3 [1,33 - 13,94]). En comparación con la VM invasiva, el fracaso de la VNI retrasó la intubación (p=0,004), prolongó la duración de la VM invasiva (p=0,007) y la estancia en la UCI (p=0,001). El fracaso de la VNI se asoció con la necesidad de recibir fármacos vasoactivos (OR 7,8 [IC 95%, 1,8 - 33,2], p=0,006).
ConclusionesEn pacientes sin EPOC con neumonía, la VNI como tratamiento ventilatorio inicial no se asoció con mejor evolución, en comparación con la VM invasiva de primera línea. El fracaso de la VNI se relacionó con duraciones más prolongadas de la MV y la estancia hospitalaria, y con mayor mortalidad hospitalaria. El uso de fármacos vasoactivos fue predictivo del fracaso de la VNI.
The use of noninvasive mechanical ventilation (NIV) in the treatment of critically ill subjects has clearly increased since the late twentieth century.1 NIV is considered part of the standard of care of subjects with COPD with acute-on-chronic respiratory failure, predominantly hypercapnic COPD exacerbations.2–4
Similarly, favorable effects of NIV have been described in COPD subjects with pneumonia,5–8 but its role in non-COPD subjects with severe pneumonia is still controversial due to high rates of NIV failure.6,9 Accordingly, there is some concern about the effects of NIV on outcome in these subjects, as lack of beneficial effects and even worse outcome have been described, especially when NIV fails,1,6,10–16 and in particular when it delays intubation.8 Several explanations, such as a delay in intubation,8 a high expired tidal volume during NIV17 or intubation complications18 have been proposed to justify the poor outcomes observed when patients need intubation after NIV failure. Furthermore, patients with severe pneumonia often have high rates of comorbidities, such as COPD, heart failure or immunosuppression,19,20 and these may have confounding effects when evaluating NIV efficacy. Despite this, NIV is commonly used in emergency departments and intensive care units (ICU) to treat pneumonia to avoid intubation.21,22
The primary objective of this study was to assess in real practice the effects of NIV on hospital mortality compared with first-line invasive MV in non-COPD patients with pneumonia that required ventilatory support. Secondary objectives were to evaluate the effects of NIV on length of stay and to identify factors that can predict NIV failure.
MethodsSetting and Study PopulationConsecutive patients admitted to our Intensive Care Unit from March 2002 to October 2012 with the principal diagnosis of pneumonia were included in the study. Subjects were identified by searching the medical report database of our ICU. Following this, each selected report was reviewed by a medical team that confirmed the diagnosis of pneumonia as the reason of ICU admission. Pneumonia was defined as pulmonary infiltrates on the chest radiograph at ICU admission together with symptoms (cough, sputum purulence, dyspnea or fever) and signs (lung crackles or leukocyte count above 12.0×109/L or below 4.0×109/L) of respiratory tract infection.23 COPD was defined as the presence of a postbronchodilator FEV1/FVC lower than 0.70. Alternatively, a clinical diagnosis of COPD was considered if the patient had a history of dyspnea, chronic cough and/or sputum production, and exposure to risk factors for the disease.24
Data were collected by consulting the electronic medical record of patients, where information on medical devices and intravenous pumps were automatically saved (Optum Clinical Solutions®).
Patients with pneumonia who did not require ventilatory support were not considered for analysis. After inclusion in the study, subjects were classified in 2 groups according to the first ventilatory treatment applied: invasive MV and NIV. Failure of NIV was defined as the need for pneumonia-related intubation at any time. Early failure of NIV was defined as the need for intubation within the first 48h of application.25
All subjects received the usual treatment given in our unit. The need for ventilatory support was decided by the attending intensivist, who chose which ventilatory support to apply (invasive or noninvasive) at his or her own discretion. The interface used in all noninvasive ventilation episodes was an oronasal mask. We followed sedation-analgesia and weaning protocols for all intubated subjects.
Data CollectionWe collected the following variables: age, gender, SAPS 3,26 date of admission and discharge from hospital and ICU, need and timing of NIV or invasive MV, death, the existence of do-not-intubate orders at ICU admission, comorbidities (COPD, diabetes mellitus, congestive heart failure, chronic renal failure, chronic liver failure, alcoholism, immunosuppression or HIV infection), previous hospital admission in the last year, physiological characteristics at admission prior to ventilation (temperature, mean arterial pressure, respiratory rate, heart rate, Glasgow coma score), laboratory blood tests at admission (leukocyte and platelet count, hemoglobin, lactate, urea, creatinine, bilirubin, albumin) and arterial blood gas analysis (pH, PaCO2, PaO2) at hospital admission, before the start of ventilatory treatment. Other variables were also collected: number of chest X-ray quadrants affected at ICU admission, initial FiO2, origin of pneumonia (community or nosocomial), use of vasoactive drugs during more than 1h during the first 24h, positive respiratory culture or positive detection of urinary antigen test by enzyme immunoassay for Streptococcus pneumonia or Legionella pneumophila, adequacy of initial antibiotic regimen. CURB65 score was also calculated.27
Ethical AspectsThe local institutional review board of Hospital Son Llàtzer (Palma de Mallorca, Spain) approved this historical cohort study in December of 2014 and waived the requirement for consent due to the characteristics of the study design.
Statistical AnalysisQualitative or categorical variables, expressed as numbers and percentages, were compared using the Chi-square test. Continuous variables were expressed as mean and standard deviation (SD) and compared between groups using the Student-t test for independent data if normality of distribution was assumed with the Kolmogorov–Smirnov test. Quantitative variables, meanwhile, were expressed as median and interquartile range [IQR] and compared using the Mann–Whitney-U test. All analyses were two-tailed, and P values ≤.05 were considered significant.
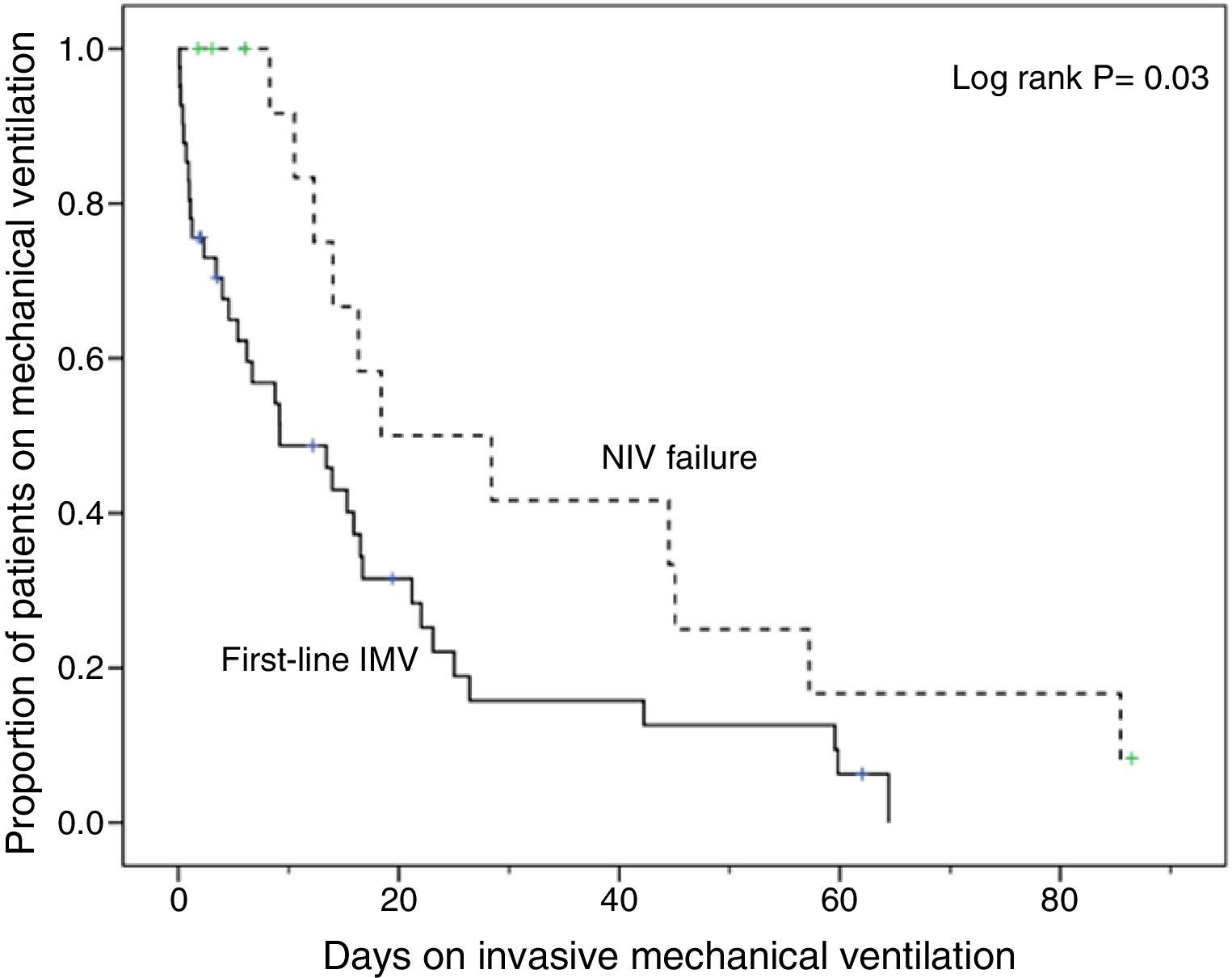
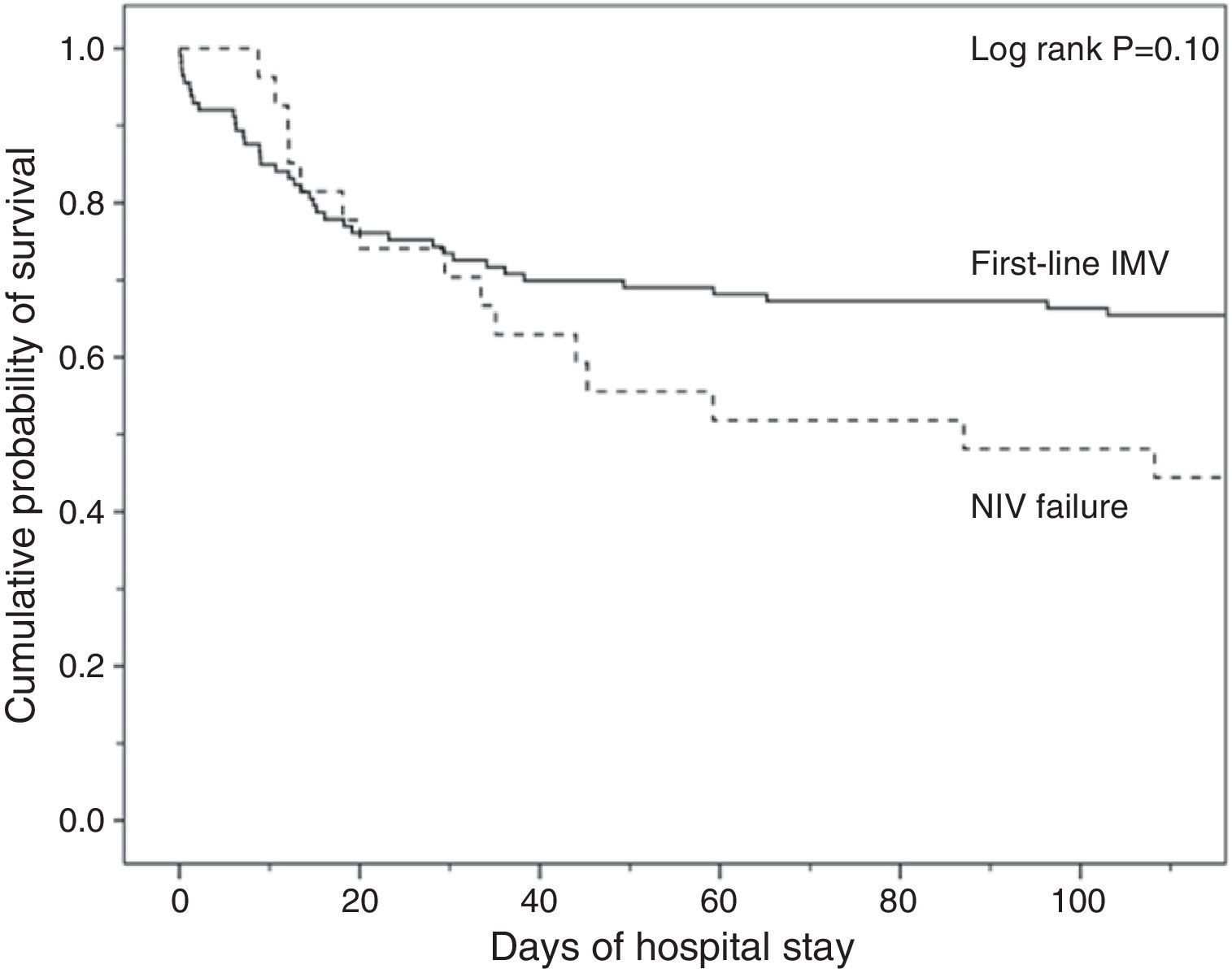
Kaplan–Meier curves of the cumulative probability of survival or the probability of remaining on mechanical ventilation according to the ventilatory treatment applied were plotted over time. Groups were compared by means of the log-rank test.
For the multivariable analysis, ventilatory support was entered as a single variable with 3 different categories: invasive MV was the reference category with an adjusted odds ratio of 1, and was compared to NIV success and NIV failure.
Considering that associations in unadjusted comparisons in an observational study could be misleading, a propensity score analysis was performed to minimize biased estimates of the initial ventilatory treatment effect. The propensity score in our study was defined, among subjects treated with invasive MV or NIV, as the conditional probability of being intubated at a first attempt given the individual's covariates, and was calculated using a multiple logistic regression model. The predictive capacity of the final model was assessed with a receiver-operating characteristic (ROC) curve and the area under the curve (AUC) was calculated.
Ventilatory support and variables associated with mortality in the univariate analysis were included in a multivariate backward stepwise elimination logistic-regression analysis (Pin<0.10 and Pout<0.05). Once the multivariate model was constructed, we added the propensity score as an independent variable to calculate the adjusted odds of hospital mortality. Data was processed with SPSS software package, version 17.0.
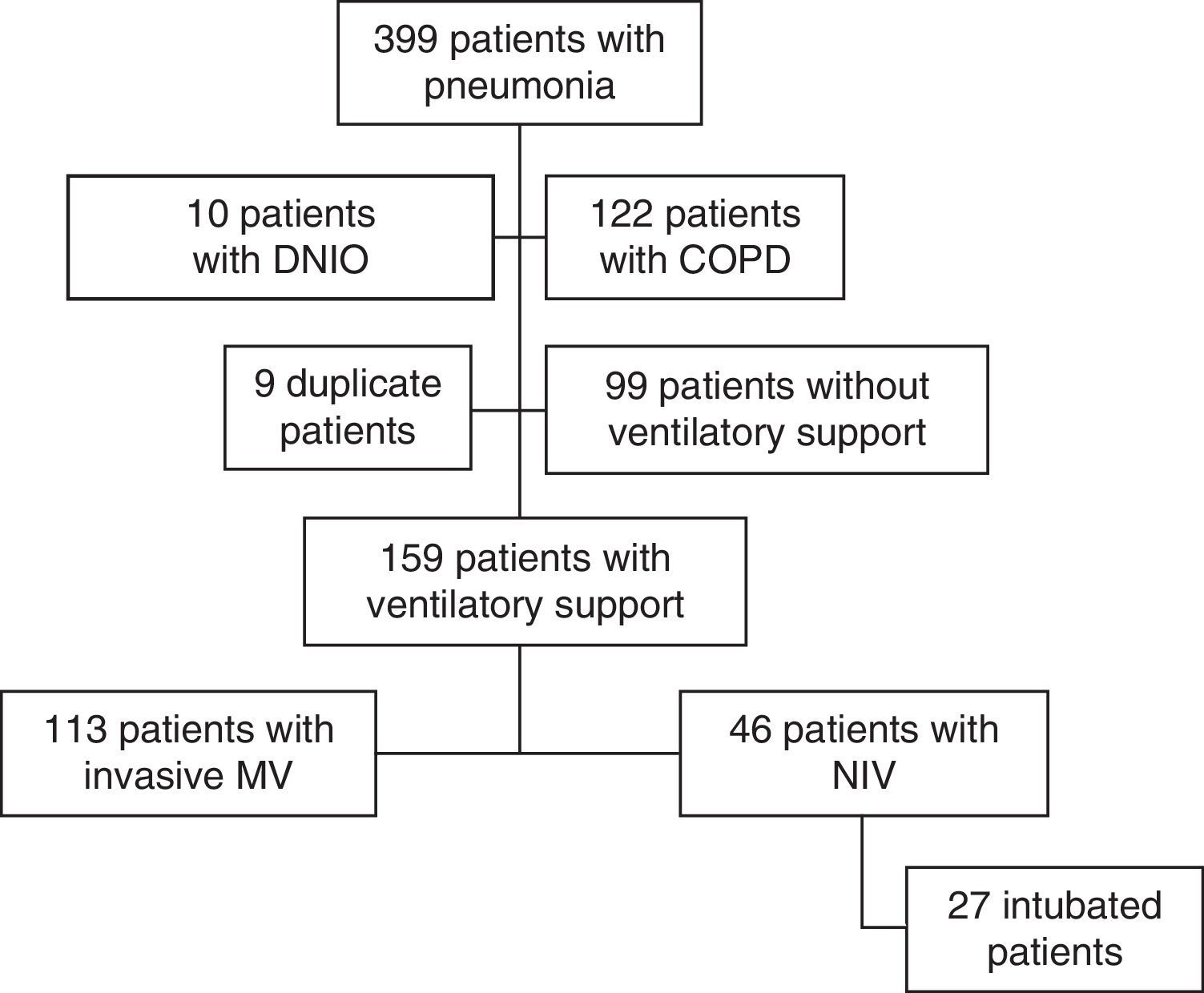
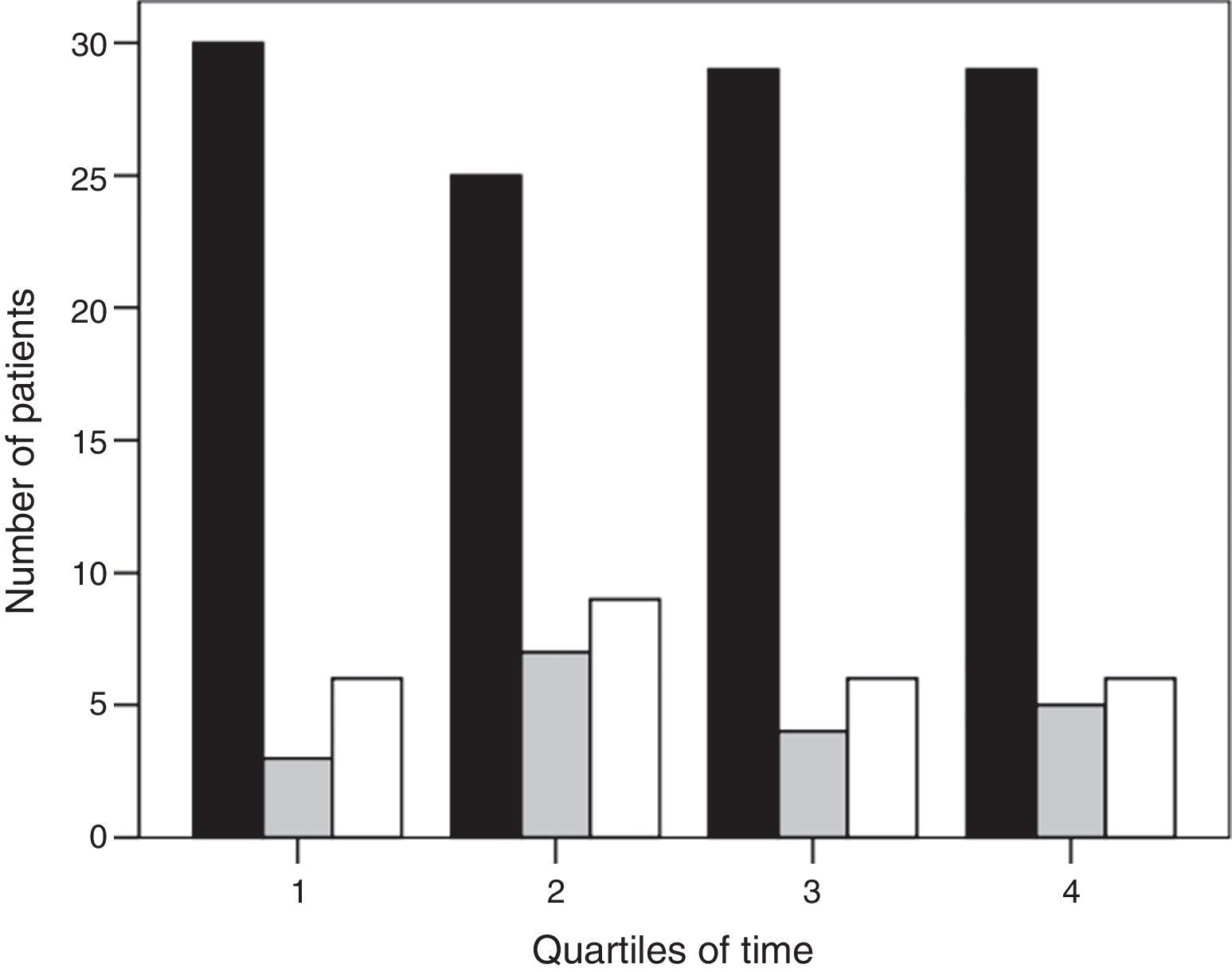
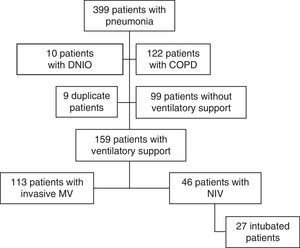
ResultsThree hundred ninety-nine subjects with pneumonia were admitted to our ICU during the study period. Fig. 1 shows the patient flow chart. One hundred fifty-nine subjects were included in the study: 113 (71%) were connected to invasive MV as initial ventilatory treatment and 46 (29%) received first-line NIV. In 27 of these 46 subjects (59%) the initial NIV attempt failed, and they were ultimately intubated. Annex 1 shows the histogram of the ventilatory treatment applied over the study period.
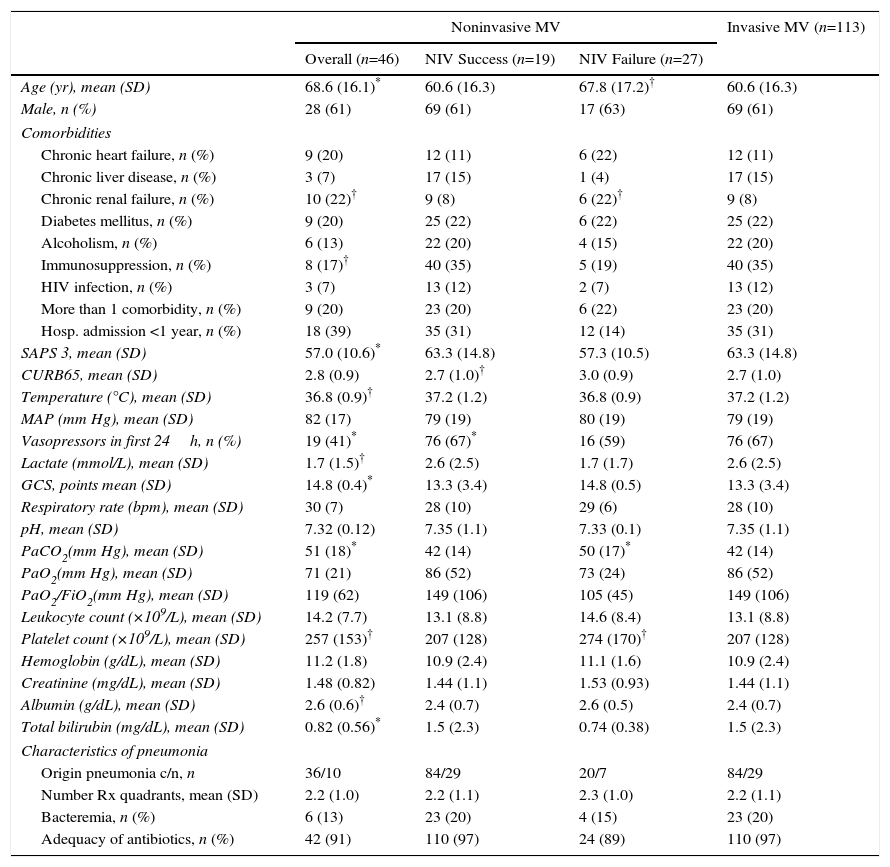
An analysis of the first ventilatory treatment applied showed that subjects with first-line invasive MV were younger, more severely ill (SAPS 3, need for vasoactive drugs, lactate levels, Glasgow coma score, albumin and total bilirubin blood levels) and had lower PaCO2 levels at ICU admission than those with first-line NIV. The first-line invasive MV group also included a lower proportion of chronic renal failure cases, and a higher proportion of immunosuppression cases (Table 1).
Demographic and Clinical Characteristics of All Non-COPD Patients With Pneumonia According to the Ventilatory Support Applied.
| Noninvasive MV | Invasive MV (n=113) | |||
|---|---|---|---|---|
| Overall (n=46) | NIV Success (n=19) | NIV Failure (n=27) | ||
| Age (yr), mean (SD) | 68.6 (16.1)* | 60.6 (16.3) | 67.8 (17.2)† | 60.6 (16.3) |
| Male, n (%) | 28 (61) | 69 (61) | 17 (63) | 69 (61) |
| Comorbidities | ||||
| Chronic heart failure, n (%) | 9 (20) | 12 (11) | 6 (22) | 12 (11) |
| Chronic liver disease, n (%) | 3 (7) | 17 (15) | 1 (4) | 17 (15) |
| Chronic renal failure, n (%) | 10 (22)† | 9 (8) | 6 (22)† | 9 (8) |
| Diabetes mellitus, n (%) | 9 (20) | 25 (22) | 6 (22) | 25 (22) |
| Alcoholism, n (%) | 6 (13) | 22 (20) | 4 (15) | 22 (20) |
| Immunosuppression, n (%) | 8 (17)† | 40 (35) | 5 (19) | 40 (35) |
| HIV infection, n (%) | 3 (7) | 13 (12) | 2 (7) | 13 (12) |
| More than 1 comorbidity, n (%) | 9 (20) | 23 (20) | 6 (22) | 23 (20) |
| Hosp. admission <1 year, n (%) | 18 (39) | 35 (31) | 12 (14) | 35 (31) |
| SAPS 3, mean (SD) | 57.0 (10.6)* | 63.3 (14.8) | 57.3 (10.5) | 63.3 (14.8) |
| CURB65, mean (SD) | 2.8 (0.9) | 2.7 (1.0)† | 3.0 (0.9) | 2.7 (1.0) |
| Temperature (°C), mean (SD) | 36.8 (0.9)† | 37.2 (1.2) | 36.8 (0.9) | 37.2 (1.2) |
| MAP (mm Hg), mean (SD) | 82 (17) | 79 (19) | 80 (19) | 79 (19) |
| Vasopressors in first 24h, n (%) | 19 (41)* | 76 (67)* | 16 (59) | 76 (67) |
| Lactate (mmol/L), mean (SD) | 1.7 (1.5)† | 2.6 (2.5) | 1.7 (1.7) | 2.6 (2.5) |
| GCS, points mean (SD) | 14.8 (0.4)* | 13.3 (3.4) | 14.8 (0.5) | 13.3 (3.4) |
| Respiratory rate (bpm), mean (SD) | 30 (7) | 28 (10) | 29 (6) | 28 (10) |
| pH, mean (SD) | 7.32 (0.12) | 7.35 (1.1) | 7.33 (0.1) | 7.35 (1.1) |
| PaCO2(mm Hg), mean (SD) | 51 (18)* | 42 (14) | 50 (17)* | 42 (14) |
| PaO2(mm Hg), mean (SD) | 71 (21) | 86 (52) | 73 (24) | 86 (52) |
| PaO2/FiO2(mm Hg), mean (SD) | 119 (62) | 149 (106) | 105 (45) | 149 (106) |
| Leukocyte count (×109/L), mean (SD) | 14.2 (7.7) | 13.1 (8.8) | 14.6 (8.4) | 13.1 (8.8) |
| Platelet count (×109/L), mean (SD) | 257 (153)† | 207 (128) | 274 (170)† | 207 (128) |
| Hemoglobin (g/dL), mean (SD) | 11.2 (1.8) | 10.9 (2.4) | 11.1 (1.6) | 10.9 (2.4) |
| Creatinine (mg/dL), mean (SD) | 1.48 (0.82) | 1.44 (1.1) | 1.53 (0.93) | 1.44 (1.1) |
| Albumin (g/dL), mean (SD) | 2.6 (0.6)† | 2.4 (0.7) | 2.6 (0.5) | 2.4 (0.7) |
| Total bilirubin (mg/dL), mean (SD) | 0.82 (0.56)* | 1.5 (2.3) | 0.74 (0.38) | 1.5 (2.3) |
| Characteristics of pneumonia | ||||
| Origin pneumonia c/n, n | 36/10 | 84/29 | 20/7 | 84/29 |
| Number Rx quadrants, mean (SD) | 2.2 (1.0) | 2.2 (1.1) | 2.3 (1.0) | 2.2 (1.1) |
| Bacteremia, n (%) | 6 (13) | 23 (20) | 4 (15) | 23 (20) |
| Adequacy of antibiotics, n (%) | 42 (91) | 110 (97) | 24 (89) | 110 (97) |
c/n: community acquired/nosocomial acquired; HIV: human immunodeficiency virus; Hosp: hospital; MV: mechanical ventilation; NIV: noninvasive mechanical ventilation; SD: standard deviation.
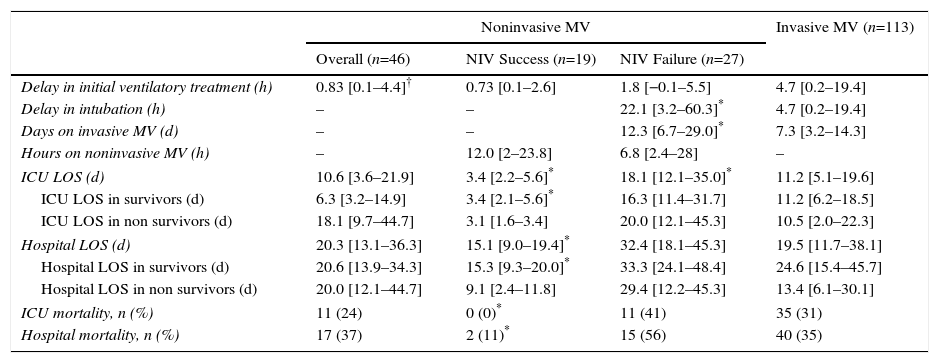
Among first-line NIV subjects, those in whom NIV failed had a higher CURB65 score compared with subjects in whom NIV succeeded; likewise, the NIV failure group included a higher proportion of cases requiring vasoactive drugs (Table 1). The use of vasoactive drugs was associated with NIV failure with an odds ratio of 7.8 (95% CI, 1.8 to 33.2), P=.006. Subjects with NIV success showed shorter length of ICU and hospital stay compared with subjects with NIV failure; duration of NIV treatment was similar in both groups (Table 2). Among subjects with NIV failure, 19 presented early failure (70%).
Outcome Characteristics of the Different Ventilatory Groups. Continuous Variables are Shown as Median and Interquartile Range.
| Noninvasive MV | Invasive MV (n=113) | |||
|---|---|---|---|---|
| Overall (n=46) | NIV Success (n=19) | NIV Failure (n=27) | ||
| Delay in initial ventilatory treatment (h) | 0.83 [0.1–4.4]† | 0.73 [0.1–2.6] | 1.8 [−0.1–5.5] | 4.7 [0.2–19.4] |
| Delay in intubation (h) | – | – | 22.1 [3.2–60.3]* | 4.7 [0.2–19.4] |
| Days on invasive MV (d) | – | – | 12.3 [6.7–29.0]* | 7.3 [3.2–14.3] |
| Hours on noninvasive MV (h) | – | 12.0 [2–23.8] | 6.8 [2.4–28] | – |
| ICU LOS (d) | 10.6 [3.6–21.9] | 3.4 [2.2–5.6]* | 18.1 [12.1–35.0]* | 11.2 [5.1–19.6] |
| ICU LOS in survivors (d) | 6.3 [3.2–14.9] | 3.4 [2.1–5.6]* | 16.3 [11.4–31.7] | 11.2 [6.2–18.5] |
| ICU LOS in non survivors (d) | 18.1 [9.7–44.7] | 3.1 [1.6–3.4] | 20.0 [12.1–45.3] | 10.5 [2.0–22.3] |
| Hospital LOS (d) | 20.3 [13.1–36.3] | 15.1 [9.0–19.4]* | 32.4 [18.1–45.3] | 19.5 [11.7–38.1] |
| Hospital LOS in survivors (d) | 20.6 [13.9–34.3] | 15.3 [9.3–20.0]* | 33.3 [24.1–48.4] | 24.6 [15.4–45.7] |
| Hospital LOS in non survivors (d) | 20.0 [12.1–44.7] | 9.1 [2.4–11.8] | 29.4 [12.2–45.3] | 13.4 [6.1–30.1] |
| ICU mortality, n (%) | 11 (24) | 0 (0)* | 11 (41) | 35 (31) |
| Hospital mortality, n (%) | 17 (37) | 2 (11)* | 15 (56) | 40 (35) |
ICU: intensive care unit; LOS: length of stay; MV: mechanical ventilation; NIV: noninvasive ventilation.
The delay in the application of the first ventilatory treatment was longer in the first-line invasive MV group compared with first-line NIV group (P=.01), with no significant differences between the NIV failure and NIV success group, or between the first-line invasive MV and NIV failure group (Table 2).
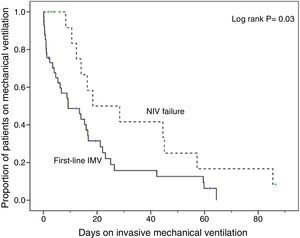
Compared with subjects treated first with invasive MV, subjects with NIV failure were older and had higher PaCO2 levels. This group also included a higher proportion of chronic renal failure cases (Table 1). In addition, they were intubated later (P<.001), had longer duration of invasive MV (P=.007) (Fig. 2) and had longer length ICU of stay (P=.001) (Table 2).
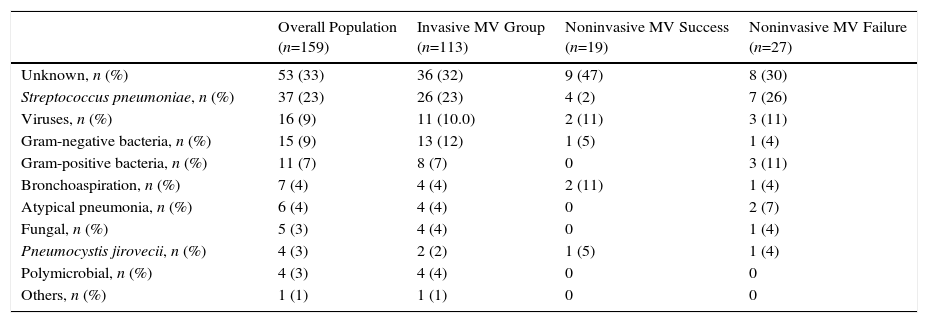
There were no significant differences among treatment groups in respect of the clinical characteristics of pneumonia (Table 1). Overall, initial antibiotic treatment was in adequate in 7 subjects (4%): 3 subjects in the invasive MV group (Pseudomonas aeruginosa, herpes simplex virus plus influenza H1N1 and herpes simplex virus), 3 in the NIV failure group (Enterococcus faecium, Aspergillus fumigatus and herpes simplex virus), and 1 in the NIV success group (influenza H1N1). Microbiological diagnoses of pneumonia are shown in Table 3.
Microbiological Diagnosis of Pneumonia of All Patients.
| Overall Population (n=159) | Invasive MV Group (n=113) | Noninvasive MV Success (n=19) | Noninvasive MV Failure (n=27) | |
|---|---|---|---|---|
| Unknown, n (%) | 53 (33) | 36 (32) | 9 (47) | 8 (30) |
| Streptococcus pneumoniae, n (%) | 37 (23) | 26 (23) | 4 (2) | 7 (26) |
| Viruses, n (%) | 16 (9) | 11 (10.0) | 2 (11) | 3 (11) |
| Gram-negative bacteria, n (%) | 15 (9) | 13 (12) | 1 (5) | 1 (4) |
| Gram-positive bacteria, n (%) | 11 (7) | 8 (7) | 0 | 3 (11) |
| Bronchoaspiration, n (%) | 7 (4) | 4 (4) | 2 (11) | 1 (4) |
| Atypical pneumonia, n (%) | 6 (4) | 4 (4) | 0 | 2 (7) |
| Fungal, n (%) | 5 (3) | 4 (4) | 0 | 1 (4) |
| Pneumocystis jirovecii, n (%) | 4 (3) | 2 (2) | 1 (5) | 1 (4) |
| Polymicrobial, n (%) | 4 (3) | 4 (4) | 0 | 0 |
| Others, n (%) | 1 (1) | 1 (1) | 0 | 0 |
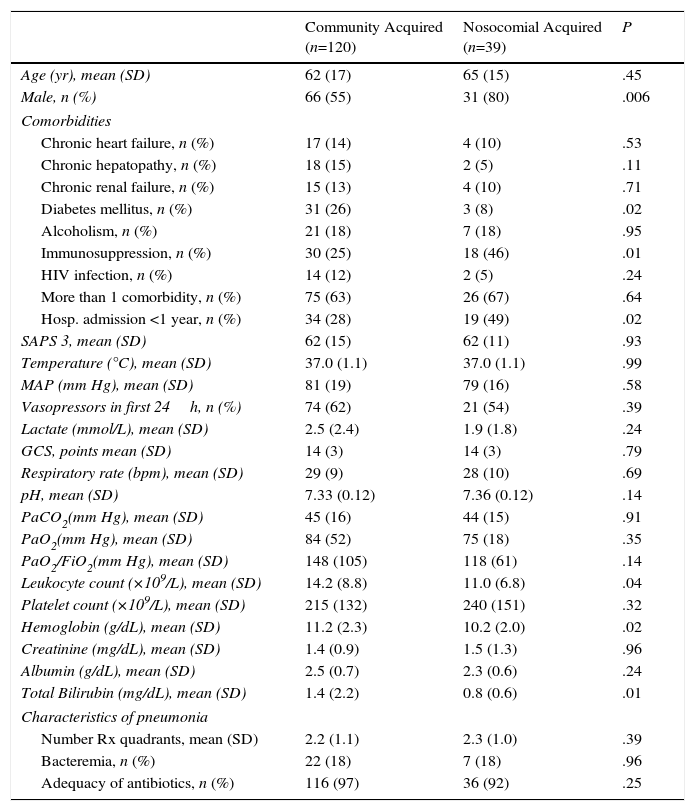
Patients with nosocomial acquired pneumonia were mainly men with more comorbidities and lower blood leukocyte count and bilirubin levels than patients with community acquired pneumonia (Annex 2), and needed longer length of stay (mean difference 17 days [95% CI, 28–7 days], P=.009).
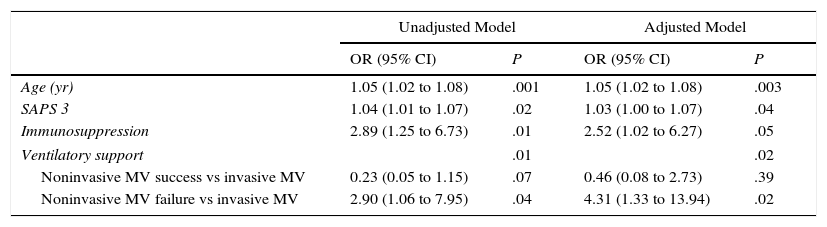
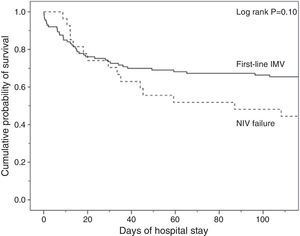
Analysis of MortalityThere was no significant difference in hospital mortality between subjects with first-line invasive MV (35%) and subjects with first-line NIV (37%) (Table 2), or between subjects with first-line invasive MV and subjects with NIV failure (56%) in the univariate analysis (Table 2) and in Kaplan–Meier curves (Fig. 3). The NIV failure group, however, had higher hospital mortality than the first-line invasive MV group. In the multivariable analysis with all ventilated subjects (invasive and noninvasive), the variables associated with hospital mortality in the final model were age, SAPS 3, immunosuppression, and NIV failure compared to first-line invasive MV (Table 4). When this model was adjusted by the propensity score of receiving first-line invasive MV or NIV as an explicative covariate, the odds of death increased 4.31 fold (95% CI, 1.33–13.94; P=.02) when subjects failed NIV compared with subjects who received first-line invasive MV (Table 4). The area under the curve of the propensity score model was 0.82 (95% CI, 0.74–0.89).
Logistic Regression Analysis of Hospital Mortality With All Ventilated Subjects. Ventilatory Support Has Been Categorized Considering First-Line Invasive MV as Reference. Data are Shown Before and After the Adjustment by the Propensity Score of Receiving Invasive MV as a First Ventilatory Treatment.
| Unadjusted Model | Adjusted Model | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age (yr) | 1.05 (1.02 to 1.08) | .001 | 1.05 (1.02 to 1.08) | .003 |
| SAPS 3 | 1.04 (1.01 to 1.07) | .02 | 1.03 (1.00 to 1.07) | .04 |
| Immunosuppression | 2.89 (1.25 to 6.73) | .01 | 2.52 (1.02 to 6.27) | .05 |
| Ventilatory support | .01 | .02 | ||
| Noninvasive MV success vs invasive MV | 0.23 (0.05 to 1.15) | .07 | 0.46 (0.08 to 2.73) | .39 |
| Noninvasive MV failure vs invasive MV | 2.90 (1.06 to 7.95) | .04 | 4.31 (1.33 to 13.94) | .02 |
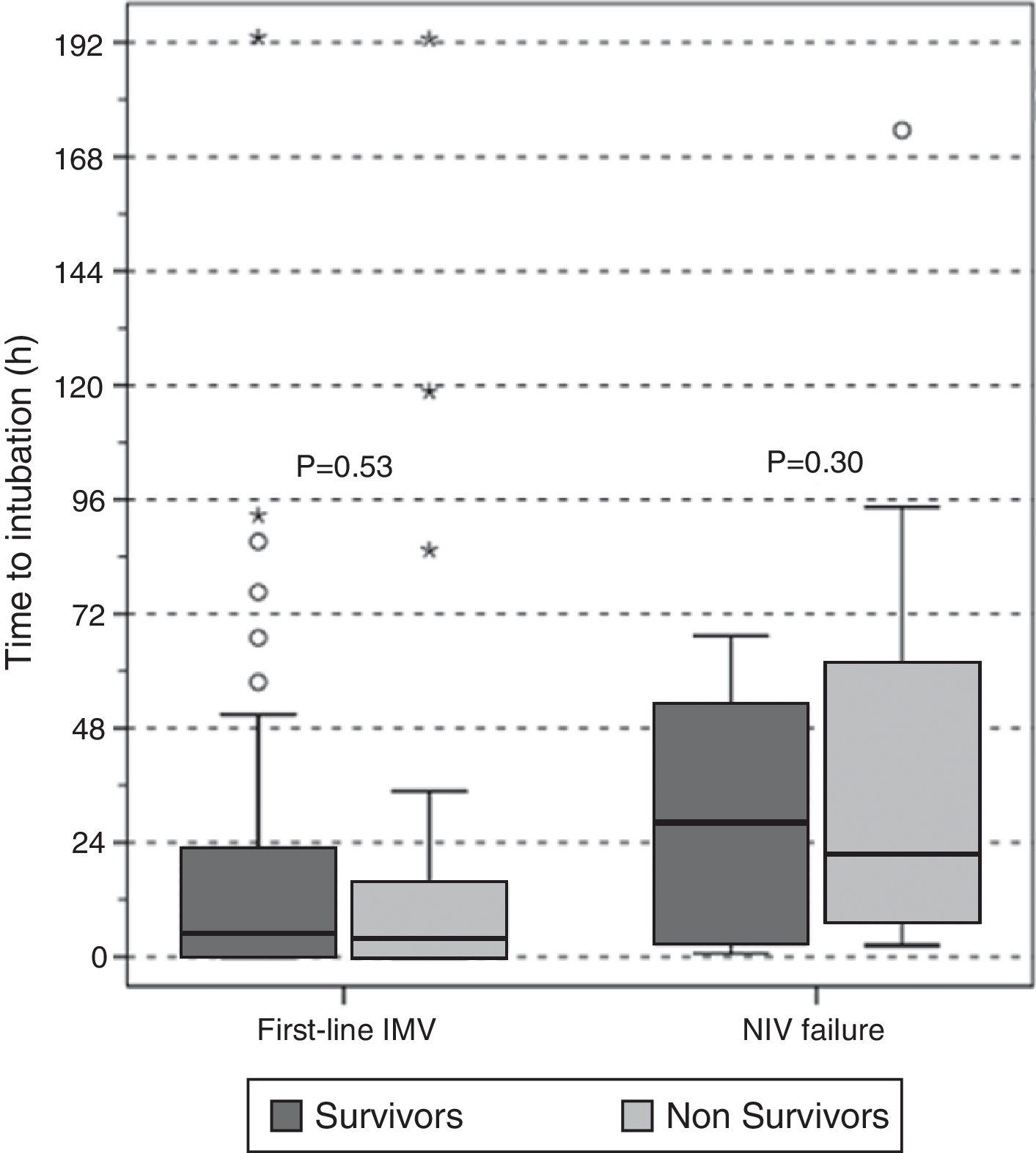
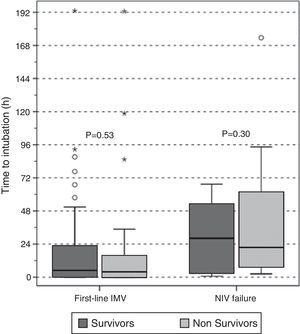
There were no differences in the delay in intubation between survivors and non-survivors in first-line invasive MV or in the NIV failure group (Fig. 4), and there was no difference in hospital mortality between subjects with early and late failure of NIV (50% vs 58%, P=.5, respectively). Moreover, there was no association between the delay in intubation and hospital mortality when overall intubated patients were evaluated (OR 1.01 [95% CI, 1.00–1.01], P=.36).
DiscussionIn our study, the application of first-line NIV in non-COPD subjects with pneumonia admitted to the ICU was not associated with a reduction in hospital stay or in hospital mortality compared with first-line invasive MV treatment. Indeed, our rate of NIV failure and need for rescue treatment with invasive MV was high and was associated with poor outcome, longer ICU and hospital stay, longer length of invasive MV and, in the adjusted multivariable analysis, greater hospital mortality compared with first-line invasive MV.
The findings of our study add to existing concerns about the potentially harmful effects of NIV failure in these subjects.1,7,12,13,18,28,29 These results deserve attention, as subjects that did not initially meet criteria for invasive MV, and a priori seemed to have a more favorable prognosis with lower SAPS 3 score, ultimately had a worse outcome than subjects directly intubated.
Since the late nineties, there has been considerable controversy regarding the effects of NIV compared to conventional oxygen therapy in subjects with acute hypoxemic respiratory failure not caused by acute heart failure or COPD exacerbation, particularly in immunocompetent subjects with pneumonia or ARDS. Some studies described beneficial effects in terms of intubation rate6,20 or mortality20; others, in contrast, warned of the potentially harmful effects of NIV failure,1,9,11–13,15,18,21,30–32 particularly in non-COPD subjects.7,9,11 These conflicting findings are probably due to differences in study design (clinical trials, surveys and case series), differences in the case mix of subjects recruited, the wide variability of severity of illness,21,33 and differences in exclusion criteria.
In order to avoid these pitfalls in our study, we excluded subjects with COPD, with do-not-resuscitate orders and with no need for ventilatory support. The exclusion of COPD and do-not-resuscitate-order subjects strengthened our results, since they are confounding factors in the analysis of the effects of NIV on outcome in subjects with pneumonia.6,34 The exclusion of subjects who did not require ventilatory support (contrary to randomized studies6,20,32) allowed us to compare the effects of the 2 ventilatory treatments when oxygen therapy plus medical treatment was deemed insufficient. In our hospital, moreover, subjects with pneumonia that required ventilatory or vasoactive support are admitted to the ICU, thus eliminating the potential bias of selecting subjects using criteria other than the decision of the attending medical team to apply ventilatory support.
PaCO2 levels were higher among patients treated with NIV compared with first-line invasive MV. As patients with COPD were excluded from the study, hypercapnia was probably due to acute ventilatory failure, since it was accompanied by mild acidosis and both groups had similar Glasgow Coma Scale scores.
Our NIV failure rate was higher than that described in clinical trials6,20,32,34,35 but consistent with the rate reported in observational studies.1,7,9–11,13 Our subjects were severely ill: 60% needed vasoactive drugs and overall hospital mortality was 36%, which is consistent with their SAPS 3 mortality prediction score.26 With regard to comorbidities, the first-line NIV group was older and more prone to chronic renal failure than the first-line invasive MV group. In contrast, SAPS 3 score and immunosuppression rate were higher in the first-line invasive MV group, suggesting that this group was more severely ill.
In spite of the prompt application of first-line ventilatory techniques, both invasive MV and NIV, subjects who failed NIV were intubated with a clinically significant delay compared with first-line invasive MV subjects.
It is important to emphasize that the NIV failure group showed higher hospital mortality compared with the invasive MV group (56% vs 35%) although this difference did not reach statistical significance, probably due to the small sample size. In some studies, delay in intubation when NIV failed was associated with an increase in mortality.8 Persistent respiratory distress during NIV treatment, with increased work of breathing ultimately leading to intubation could explain this poor outcome. Early intubation and sedation may decrease the work of breathing, dyssynchrony and biotrauma.36 High tidal volumes administered during NIV could also increase ventilator-induced lung injury17 and mortality.37 Unfortunately, we did not record physiologic or ventilatory parameters during the NIV period. Moreover, Mosier et al.18 described an increase in intubation complications in subjects with NIV failure compared with those directly intubated, and this could partially explain the increase in mortality observed in subjects with NIV failure. However, in our study no association was found between the delay in intubation and mortality. This is in line with Thille et al.,30 who observed no differences in intubation time between survivors and non-survivors, with values very close to ours, and contrasts with Carrillo et al.8 Our short delay in intubation, with 70% of subjects intubated within 48h, may explain the lack of association with increased mortality. In addition, we found no inter-group differences in the adequacy of antibiotic treatment that could explain differences in mortality, and causative organisms were not identified in over a third of subjects, as described in other studies.38 In our study, patients with NIV failure inevitably needed invasive MV, but we do not know whether some patients in the first-line invasive MV group would have succeeded with NIV. We cannot reject the hypothesis that NIV failure was a result of otherwise undetected severity (SAPS 3 or the need of vasoactive drugs), and may not in itself have worsened prognosis.
In clinical practice, one of the first decisions to be made in non-COPD subjects with severe pneumonia is whether to use invasive MV or NIV. In our study, the only variable associated with NIV failure was the use of vasoactive drugs in the first 24h of ICU admission. This is consistent with the findings of several studies.13,30 Of note, respiratory parameters associated with severity of pneumonia, such as oxygenation (measured by PaO2/FiO2), the number of radiographic lung quadrants involved or the existence of bacteremia, did not differ between subjects with successful and failed NIV.
Primary among the limitations of our study is its single-center retrospective design, which compels us to view our results with caution. Second, no protocol was in place to determine the need for ventilatory support or the type of support given, and the decision was taken at the discretion of the attending medical team. Third, we did not record the chronology of administration of the initial non-ventilatory medical treatment given, such as antibiotics or fluid replacement. Fourth, time-dependent changes in treatment policies that could have modified patient outcome over the study period, such as implementation of the Surviving Sepsis Campaign,39 were not taken into account. Fifth, the heterogeneity of the different baseline characteristics of patients in the ventilatory groups could have led to a potentially misleading interpretation of our results. To minimize these important shortcomings, we adjusted our analysis of mortality with the propensity score that defined the probability of being intubated in a first attempt given the individual's covariates, although the method used may not fully overcome bias in the allocation of treatment.40
ConclusionsIn non-COPD subjects with pneumonia and need for ventilatory support, those treated with first-line NIV did not have better outcome than subjects treated initially with invasive MV. Failure of NIV was associated with longer duration of MV, longer ICU stay and with an increase in hospital mortality compared with subjects with first-line invasive MV. The need for vasoactive drugs discourages the application of first-line NIV owing to the high rate of NIV failure observed.
AuthorshipLiterature research: Gemma Rialp, Daniel Muñiz, Maria Romero.
Data collection: Gemma Rialp, Catalina Forteza, Daniel Muñiz, Maria Romero.
Study design: Gemma Rialp.
Data analysis: Gemma Rialp.
Manuscript preparation: Gemma Rialp, Catalina Forteza.
Review of manuscript: Daniel Muñiz, Maria Romero.
FundingNo funding was received for this study.
Conflict of InterestThe authors declare that they have no conflict of interest.
| Community Acquired (n=120) | Nosocomial Acquired (n=39) | P | |
|---|---|---|---|
| Age (yr), mean (SD) | 62 (17) | 65 (15) | .45 |
| Male, n (%) | 66 (55) | 31 (80) | .006 |
| Comorbidities | |||
| Chronic heart failure, n (%) | 17 (14) | 4 (10) | .53 |
| Chronic hepatopathy, n (%) | 18 (15) | 2 (5) | .11 |
| Chronic renal failure, n (%) | 15 (13) | 4 (10) | .71 |
| Diabetes mellitus, n (%) | 31 (26) | 3 (8) | .02 |
| Alcoholism, n (%) | 21 (18) | 7 (18) | .95 |
| Immunosuppression, n (%) | 30 (25) | 18 (46) | .01 |
| HIV infection, n (%) | 14 (12) | 2 (5) | .24 |
| More than 1 comorbidity, n (%) | 75 (63) | 26 (67) | .64 |
| Hosp. admission <1 year, n (%) | 34 (28) | 19 (49) | .02 |
| SAPS 3, mean (SD) | 62 (15) | 62 (11) | .93 |
| Temperature (°C), mean (SD) | 37.0 (1.1) | 37.0 (1.1) | .99 |
| MAP (mm Hg), mean (SD) | 81 (19) | 79 (16) | .58 |
| Vasopressors in first 24h, n (%) | 74 (62) | 21 (54) | .39 |
| Lactate (mmol/L), mean (SD) | 2.5 (2.4) | 1.9 (1.8) | .24 |
| GCS, points mean (SD) | 14 (3) | 14 (3) | .79 |
| Respiratory rate (bpm), mean (SD) | 29 (9) | 28 (10) | .69 |
| pH, mean (SD) | 7.33 (0.12) | 7.36 (0.12) | .14 |
| PaCO2(mm Hg), mean (SD) | 45 (16) | 44 (15) | .91 |
| PaO2(mm Hg), mean (SD) | 84 (52) | 75 (18) | .35 |
| PaO2/FiO2(mm Hg), mean (SD) | 148 (105) | 118 (61) | .14 |
| Leukocyte count (×109/L), mean (SD) | 14.2 (8.8) | 11.0 (6.8) | .04 |
| Platelet count (×109/L), mean (SD) | 215 (132) | 240 (151) | .32 |
| Hemoglobin (g/dL), mean (SD) | 11.2 (2.3) | 10.2 (2.0) | .02 |
| Creatinine (mg/dL), mean (SD) | 1.4 (0.9) | 1.5 (1.3) | .96 |
| Albumin (g/dL), mean (SD) | 2.5 (0.7) | 2.3 (0.6) | .24 |
| Total Bilirubin (mg/dL), mean (SD) | 1.4 (2.2) | 0.8 (0.6) | .01 |
| Characteristics of pneumonia | |||
| Number Rx quadrants, mean (SD) | 2.2 (1.1) | 2.3 (1.0) | .39 |
| Bacteremia, n (%) | 22 (18) | 7 (18) | .96 |
| Adequacy of antibiotics, n (%) | 116 (97) | 36 (92) | .25 |
HIV: human immunodeficiency virus; SD: standard deviation.
Please cite this article as: Rialp G, Forteza C, Muñiz D, Romero M. El papel de la ventilación no invasiva como tratamiento ventilatorio inicial en pacientes con neumonía y sin enfermedad pulmonar obstructiva crónica. Arch Bronconeumol. 2017;53:480–488.