In the last decades, video-assisted thoracic surgery (VATS) has been proven to be feasible, safe, and oncologically equivalent to traditional thoracotomy and it offers several advantages to patients compared to open approaches.1 Therefore, it has become the standard of care for patients with operable pulmonary malignancies. As an alternative minimally invasive technique, robot-assisted thoracic surgery (RATS) has recently emerged for the treatment of patients requiring thoracic surgery.
Studies comparing RATS and VATS in terms of postoperative outcomes have shown contradictory clinical results.2–4 Additionally, RATS is frequently related to higher costs when compared to VATS.4,5 Nevertheless, to date, no studies have focused on how to maximize the advantages of RATS by recognizing those patients who would benefit more from such approach.
On the other hand, body mass index (BMI) has been associated to postoperative outcomes after lung resection for cancer, but the results are still conflicting6–9 and the impact of minimally invasive surgery in patients with different BMI ranges has not been fully characterized.
The aim of this study is to investigate if the type of minimally invasive approach (RATS or VATS) influences the occurrence of postoperative complications in anatomical lung resection according to patient's BMI.
A single-centre observational, retrospective, and comparative cohort study was conducted. All data were obtained from an institutional prospective, computerized database. Definition of variables was standardized.10 The inclusion criteria consisted of patients ages≥18 years who underwent minimally invasive anatomic lung resection (segmentectomy, lobectomy, bilobectomy) from June 2018 to January 2022 according to standardized selection criteria.11 Patients who underwent pneumonectomy or with missing data were excluded.
The choice of minimally invasive surgical approach (VATS or RATS) was mainly based on robot equipment availability, but not on tumour or patients’ characteristics. All VATS procedures were performed by a team of five board certified thoracic surgeons. RATS anatomical resections were performed by two members of the same team who were Intuitive Surgical certified surgeons. Operative technique was standardized for both approaches as previously described.12 Perioperative management was uniform for all patients throughout the study period.
Primary and secondary outcomes. The primary endpoint was overall morbidity, defined as any adverse event occurred within 30 days after the operation, or later if the patient was still in the hospital. Secondary endpoints were cardiopulmonary and major complications. Cardiopulmonary complications included respiratory failure, need for reintubation, prolonged mechanical ventilation>24h, pneumonia, atelectasis requiring bronchoscopy, pulmonary oedema, pulmonary embolism, acute respiratory distress syndrome/acute lung injury, arrhythmia requiring treatment, acute myocardial ischaemia, acute cardiac failure, stroke/transient ischaemic attack and acute kidney injury. Postoperative complications were classified according to the Dindo et al.13 and included as a binary variable (major, classes IIIA–V, or minor complications, classes I and II).
Propensity score matching. RATS and VATS cases were matched by propensity score matching (PSM) to ensure an even distribution of confounders between groups. The 1:1 nearest neighbour matching without replacement method was applied. The variables used for PSM were selected according to their clinical relevance and statistical significance: age, sex, BMI, ppoFEV1%, ppoDLCO% and tumour size.
Statistical analysis. Categorical variables were compared using the Pearson χ2 test or Fisher's exact test. Normally distributed continuous variables are presented as the mean±standard deviation (SD), and Student's t test was used for comparisons. For continuous variables that were not normally distributed, data are presented as the median (interquartile range [IQR]) and were compared by the Mann–Whitney U test between the groups. The test level between the two groups was set at α=0.05 (bilateral), and a two-sided p<0.05 was considered statistically significant. Subgroup analyses were performed for the perioperative outcomes according to BMI ranges. For this purpose, patients were divided into two groups according to BMI: under and normal weight group (BMI<25kg/m2) and overweight and obesity group (BMI≥25kg/m2).
The MatchIt package of R software, v4.2.2 (The R Foundation for Statistical Computing, Vienna, Austria) was used for PSM, and SPSS software, v26.0 (SPSS Inc., Chicago, IL, USA) was used for further data analysis.
Our manuscript is reported according to the STROBE recommendations.
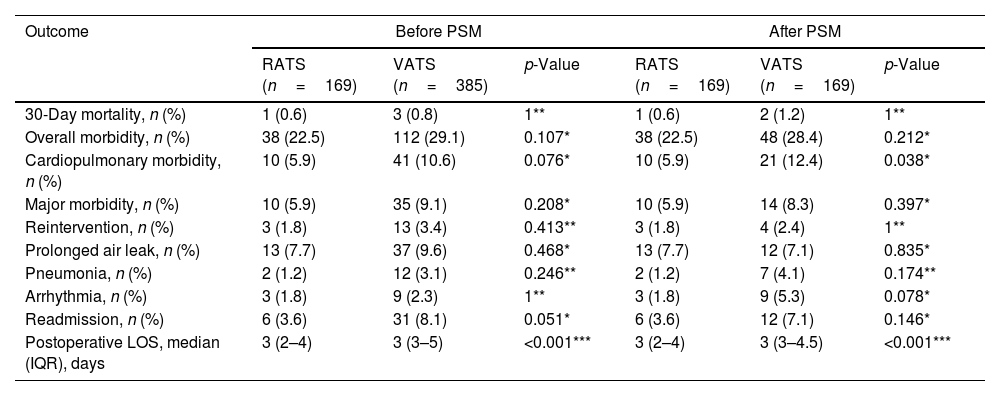
A total of 554 patients (385 VATS cases and 169 RATS cases) were included. After PSM, 169 VATS and 169 RATS patients were analyzed. There were no significant differences in any variables among groups. Table 1 shows perioperative outcomes of RATS and VATS before and after PSM. No statistically differences among group were found before matching regarding the evaluated outcomes. After PSM, the incidence of cardiopulmonary complications was significantly reduced in the RATS group (12.4% vs 5.9%, p=0.038). No statistically significant difference in the rates of overall morbidity, major complications, prolonged air leak, pneumonia and arrhythmia was found.
Perioperative outcomes of RATS and VATS before and after PSM.
| Outcome | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| RATS (n=169) | VATS (n=385) | p-Value | RATS (n=169) | VATS (n=169) | p-Value | |
| 30-Day mortality, n (%) | 1 (0.6) | 3 (0.8) | 1** | 1 (0.6) | 2 (1.2) | 1** |
| Overall morbidity, n (%) | 38 (22.5) | 112 (29.1) | 0.107* | 38 (22.5) | 48 (28.4) | 0.212* |
| Cardiopulmonary morbidity, n (%) | 10 (5.9) | 41 (10.6) | 0.076* | 10 (5.9) | 21 (12.4) | 0.038* |
| Major morbidity, n (%) | 10 (5.9) | 35 (9.1) | 0.208* | 10 (5.9) | 14 (8.3) | 0.397* |
| Reintervention, n (%) | 3 (1.8) | 13 (3.4) | 0.413** | 3 (1.8) | 4 (2.4) | 1** |
| Prolonged air leak, n (%) | 13 (7.7) | 37 (9.6) | 0.468* | 13 (7.7) | 12 (7.1) | 0.835* |
| Pneumonia, n (%) | 2 (1.2) | 12 (3.1) | 0.246** | 2 (1.2) | 7 (4.1) | 0.174** |
| Arrhythmia, n (%) | 3 (1.8) | 9 (2.3) | 1** | 3 (1.8) | 9 (5.3) | 0.078* |
| Readmission, n (%) | 6 (3.6) | 31 (8.1) | 0.051* | 6 (3.6) | 12 (7.1) | 0.146* |
| Postoperative LOS, median (IQR), days | 3 (2–4) | 3 (3–5) | <0.001*** | 3 (2–4) | 3 (3–4.5) | <0.001*** |
RATS: robotic-assisted thoracoscopic surgery; VATS: video-assisted thoracoscopic surgery; LOS: length of hospital stay; IQR: interquartile range.
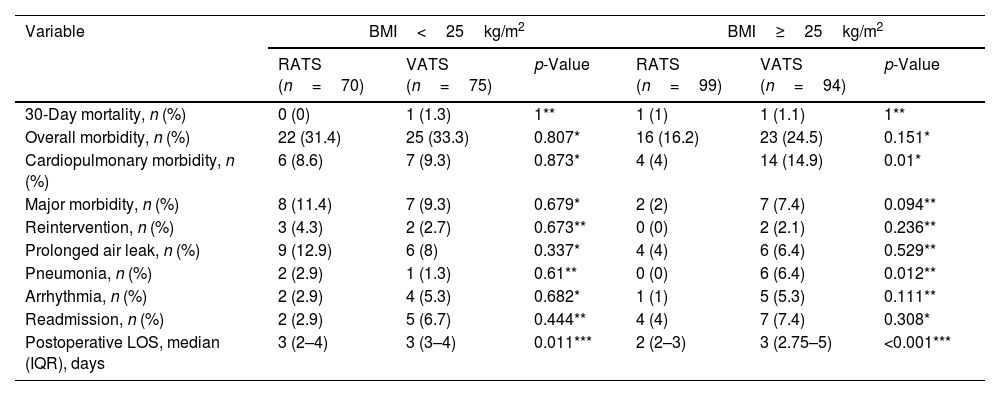
Table 2 shows subgroup analysis of postoperative outcomes between RATS and VATS according to BMI ranges after PSM. The incidence of cardiopulmonary and pneumonia in the RATS group for patients with BMI≥25kg/m2 was significantly lower, but not in the BMI<25kg/m2 group.
Subgroup analysis of postoperative outcomes between RATS and VATS according to BMI ranges after PSM.
| Variable | BMI<25kg/m2 | BMI≥25kg/m2 | ||||
|---|---|---|---|---|---|---|
| RATS (n=70) | VATS (n=75) | p-Value | RATS (n=99) | VATS (n=94) | p-Value | |
| 30-Day mortality, n (%) | 0 (0) | 1 (1.3) | 1** | 1 (1) | 1 (1.1) | 1** |
| Overall morbidity, n (%) | 22 (31.4) | 25 (33.3) | 0.807* | 16 (16.2) | 23 (24.5) | 0.151* |
| Cardiopulmonary morbidity, n (%) | 6 (8.6) | 7 (9.3) | 0.873* | 4 (4) | 14 (14.9) | 0.01* |
| Major morbidity, n (%) | 8 (11.4) | 7 (9.3) | 0.679* | 2 (2) | 7 (7.4) | 0.094** |
| Reintervention, n (%) | 3 (4.3) | 2 (2.7) | 0.673** | 0 (0) | 2 (2.1) | 0.236** |
| Prolonged air leak, n (%) | 9 (12.9) | 6 (8) | 0.337* | 4 (4) | 6 (6.4) | 0.529** |
| Pneumonia, n (%) | 2 (2.9) | 1 (1.3) | 0.61** | 0 (0) | 6 (6.4) | 0.012** |
| Arrhythmia, n (%) | 2 (2.9) | 4 (5.3) | 0.682* | 1 (1) | 5 (5.3) | 0.111** |
| Readmission, n (%) | 2 (2.9) | 5 (6.7) | 0.444** | 4 (4) | 7 (7.4) | 0.308* |
| Postoperative LOS, median (IQR), days | 3 (2–4) | 3 (3–4) | 0.011*** | 2 (2–3) | 3 (2.75–5) | <0.001*** |
BMI: body mass index; RATS: robotic-assisted thoracoscopic surgery; VATS: video assisted thoracoscopic surgery; LOS: length of postoperative hospital stays; IQR: interquartile range.
The results of our study suggest that RATS might be superior to VATS in decreasing postoperative cardiopulmonary morbidity. These findings are consistent with those reported in two recently published meta-analysis2,4 but contradictory to those reported by others authors who did not find differences in postoperative morbidity rates among both approaches.3,14
Interestingly, we also found that the incidence of cardiopulmonary and pneumonia was significantly reduced in patients≥25kg/m2 approached by RATS. Therefore, selecting RATS in this subset of patients requiring anatomical lung resection could be considered cost-effective.
Our findings are comparable to those published by Li et al.15 who found a lower incidence of overall morbidity in patients with BMI≥24kg/m2 undergoing robotic lobectomy. Similarly, Qu et al.16 found that for NSCLC patients with 24kg/m2<BMI<28kg/m2, RATS may provide better perioperative outcomes in terms of higher number of lymph nodes dissected and lower perioperative complications. However, in that study, for obese patients (BMI≥28kg/m2), RATS did not achieve better outcomes than VATS.
We speculate that RATS is associated to lower morbidity rates due to several reasons. First, the CO2 insufflation used during RATS approach allows thoracic surgeons to achieve a better view of the surgical field. The pressure of CO2 pushes down on the diaphragm and compresses the pulmonary parenchyma, increasing the surgeon's workspace which may be quite limited in obese patients. Second, the increased pressure in the chest cavity may also effectively reduce the mild bleeding of the tissues.
Certain limitations constrain the broad interpretation of our data. Firstly, its retrospective nature is a limitation of our study. Additionally, data were obtained from a single public institution with standardized clinical pathways. Secondly, although the distribution of confounder variables among RATS and VATS groups was controlled by PSM, potential selection bias could not be fully eliminated. Therefore, our results should be confirmed in other patient cohorts from different multi-institutional databases.
In our series, RATS approach for anatomical lung resection is followed by a lower incidence of postoperative cardio-respiratory complications compared to VATS in patients with ≥25kg/m2.
Funding statementThe authors declare that no financial support was received regarding the content of this manuscript.
Conflict of interestThe authors declare that there is no conflict of interest regarding the content of this manuscript.