One-year survival in lung transplant is around 85%, but this figure has not increased in recent years, in spite of technical improvements.
MethodsRetrospective, multicenter cohort study. Data from 272 eligible adults with lung transplant were recorded at 7 intensive care units (ICU) in Spain in 2013. The objective was to identify variables that might help to guide future clinical interventions in order to reducethe risk of death in the postoperative period.
ResultsOne patient (0.3%) died in the operating room and 27 (10%) within 90 days. Twenty (7.4%) died within 28 days, after a median of 14 ICU days. Grade 3 pulmonary graft dysfunction was documented in 108 patients, of whom 21 died, compared with 6 out of 163 without pulmonary graft dysfunction (P<.001). At ICU admission, non-survivors had significantly lower (P=.03) median PaO2/FiO2 (200mmHg vs 280mmHg), and the difference increased after 24hours (178 vs 297mmHg, P<.001). Thirteen required extracorporeal membrane oxygenation, and 7(53.8%) died. A logistic regression model identified pulmonary graft dysfunction (OR: 6.77), donor age>60yr (OR: 2.91) and SOFA>8 (OR: 2.53) as independent predictors of 90-day mortality. At ICU admission, higher median procalcitonin (1.6 vs 0.6) and lower median PaO2/FiO2 (200 vs 280mmHg) were significantly associated with mortality.
ConclusionGraft dysfunction remains a significant problem in lung transplant. Early ICU interventions in patients with severe hypoxemia or high procalcitonin are crucial in order to lower mortality.
La supervivencia anual del trasplante de pulmón está alrededor del 85% y este porcentaje no se ha incrementado recientemente, a pesar de mejoras técnicas.
MétodosEstudio de cohortes, multicéntrico, retrospectivo. Se recogieron datos de 272 adultos con trasplante de pulmón en 7 unidades de cuidados intensivos españolas en 2013. El objetivo fue identificar variables que pudieran ser de utilidad para guiar futuras intervenciones clínicas para disminuir el riesgo de fallecer en el postoperatorio.
ResultadosUn paciente (0,3%) falleció en quirófano y 27 (10%) a los 90 días. Veinte (7,4%) fallecieron en 28 días, después de una mediana de 14 días en unidad de cuidados intensivos. La disfunción primaria grado 3 se documentó en 108 pacientes, de los cuales 21 fallecieron, comparado con 6 de 163 sin disfunción primaria grado 3 (p<0,001). Al ingreso en unidad de cuidados intensivos, los no supervivientes mostraban una significativa menor mediana (p=0,03) de PaO2/FiO2 (200 vs. 280mmHg); esta diferencia se incrementó a las 24h (178 vs. 297mmHg, p<0,001). Trece requirieron oxigenación con membrana extracorpórea (53,8%) y 7 fallecieron. Un modelo de regresión logística múltiple identificó la disfunción primaria grado 3 (OR: 6,77), edad donante>60 años (OR: 2,91) y SOFA>8 (OR: 2,53) como predictores independientes (p<0,05) de mortalidad a los 90 días. En el ingreso en unidad de cuidados intensivos, una mediana de procalcitonina plasmática superior (1,6 vs. 0.6ng/mL) e inferior de PaO2/FiO2 (200 vs. 280mmHg) se asociaron independientemente (p<0,05) con la mortalidad.
ConclusiónLa disfunción primaria del injerto continúa siendo un problema significativo en el trasplante pulmonar. Las intervenciones precoces dirigidas a mejorar la hipoxemia o la identificación de elevación de procalcitonina representan oportunidades para disminuir la mortalidad.
Adult patients undergoing primary lung transplantation have unadjusted survival rates of 89% at 3 months, 80% at 1 year, 65% at 3 years, 54% at 5 years and 31% at 10 years.1 The most frequently reported causes of death within the first 30 days are graft failure and non-cytomegalovirus (CMV) infections. Over the first year post-transplant, non-CMV infections are the major cause of death. After this time, bronchiolitis, graft failure, non-CMV infections and malignancy become important contributors to mortality. The primary objective of this study was to improve our understanding of the interaction between donor variables, recipients, surgical conditions, and immediate postoperative management variables and their effect on ICU survival in patients with LT. The secondary objective was to identify possible biomarkers of mortality in the immediate postoperative period.
MethodsThis is an analysis of a specific database which recorded all lung transplants performed in 2013 in Spain. Only adult patients who survived the intervention and for whom complete donor and recipient data were available were eligible for analysis of prognostic factors. This multicenter study was performed at 7 large general referral university hospitals (>700 beds) with active lung transplant programs. Ethical approval was obtained and the need for informed consent was waived at each center due to the observational nature of the study. All cases were recorded continuously.
DefinitionsThe APACHE (Acute Physiology And Chronic Health Evaluation) II score was designed to measure severity of disease in adult patients admitted to intensive care units.2 It generates a point score ranging from 0 to 71 based on 12 physiological variables (including PaO2, respiratory rate, heart rate, hematocrit and creatinine), age, and underlying health. The SOFA (Sequential Organ Failure Assessment) score is a composite score comprising 6 items: the respiratory, coagulation, hepatic, cardiovascular, renal and neurological systems. It is used to track patient status during ICU stay and to establish the extent of organ function or failure.3
Pulmonary graft dysfunction (PGD) severity was graded according to the International Society for Heart and Lung Transplantation (ISHLT) consensus,4 using the partial pressure of arterial oxygen (PaO2) to fraction of inspired oxygen (FiO2) ratio. Grade 3 PGD was defined as a PaO2/FiO2 ratio of <200mmHg.
Pneumonia was defined as new or progressive radiographic opacity, ≥104 colony forming units·mL−1 in bronchoalveolar lavage (BAL) fluid and at least 2of the following: fever >38°C, leukocytosis (≥10,000cellsmm−3) or leukopenia (<4000cellsmm−3) plus purulent secretions.5 Acute rejection was determined using the ISHLT consensus.6,7
BiomarkersBlood samples were collected in sterile ethylenediaminetetraacetic acid tubes (Vacutainer; Becton Dickinson, Cockeysville, MD). Tubes were immediately centrifuged at 915,642×g for 10min at 48°C, and serum was kept refrigerated at −80°C until assayed. Plasma procalcitonin serum concentration (PCT) was determined by immunofluorescent assay, using an automated sandwich-type immunoanalysis (Thermo Scientific™ BRAHMS™ KRYPTOR™; Hennigsdorf, Germany) with TRACE (Time-Resolved Amplified Cryptate Emission) technology.
Statistical AnalysisAll comparisons were unpaired. All tests of significance were two-tailed, and P values of .05 or less were regarded as indicating statistical significance. Continuous variables were compared using the Student's t test or ANOVA for normally distributed variables, and the Mann–Whitney U or Kruskal–Wallis test for non-normally distributed variables. The chi-square or Fisher's exact test was used to compare categorical variables. Values were expressed as medians (25th–75th percentile) (continuous variables) or as a frequency of occurrence in the group from which they were derived (categorical variables).
Multivariate stepwise logistic regression was performed with 90-day (and 28-day) mortality being the dependent variables. Variables found in less than 10% of the sample were not entered in the multivariate models to avoid instability. All remaining covariates which were statistically significant at 0.05 in the univariate analysis were included in the model. A second model with 28-day mortality was also assessed. All statistical analyses were performed using SPSS, version 21.0 for Windows (SPSS Inc., Chicago, IL).
ResultsTwo hundred and eighty-five lung transplants were performed over the study period. One hundred forty-nine (54.8%) bilateral and 123 (45.2%) unilateral transplants were eligible for the study. One patient (0.3%) died in the operating room, and was excluded from analysis. Twenty-seven (10.8%) died within 90 days. Twenty (7.4%) died within 28 days after a median of 14 ICU days. Two hundred and thirty-eight survived and were discharged from the ICU after a median of 7days. Mean age was 53.2 years, and 165 (61.3%) were males. Median body mass index was 25.0. Interstitial (43.4%), obstructive (40.4%) and suppurative diseases (15.8%) were the main underlying conditions. Twelve (4.4%) patients had primary pulmonary hypertension. Chronic bacterial colonization was documented in 104 (38.5%). With the exception of donor age (60-yr cut-off point), none of the variables were significantly associated with mortality.
In donors, median ICU stay was 2 days, and many donor variables were associated with overall mortality: non-survivors had similar donor age (median 54.1 vs 50.0 yr), PaO2/FiO2 ratio (median 447.5 vs 450mmHg), smoking history (12.8% vs 9.8%), diabetes (8.3% vs 8.7%), cause of death, presence of opacities in chest radiograph (9.6% vs 10.9%), and normal bronchoscopy (89.1% vs 89.7%). In donors aged over 60 years (24.4%), 90-day mortality was significantly higher (16.6% vs 8.2%).
Regarding surgical issues, no significant differences were reported for ischemia time (320 vs 345min), use of cardiopulmonary bypass (12.2% vs 10%) or median surgical time (6.0 vs 6.3h). Significantly higher mortality was recorded in the 89 (32.7%) patients who underwent posterolateral thoracotomy (OR: 2.65. 95%CI 1.20–5.85). Only 3 patients required lobectomy due to discordant size, one of whom died. Several centers in our study perform different thoracotomies for single and bilateral lung transplant (SLT and BLT respectively). One site carried out 2 anterolateral thoracotomies for BLT, and another used clamshell thoracotomy; due to this variability, anterolateral thoracotomy was not considered a mortality factor. Basiliximab was prescribed as an induction immunosuppressor in 106 (30.1%) patients, and was associated with a trend toward lower mortality (OR: 0.41, 95%CI 0.16–1.06). Re-intervention after ICU admission was required in 25 (9.3%) patients; this subset presented increased mortality (32%) with OR: 5.55 (95%CI 2.12–14.52).
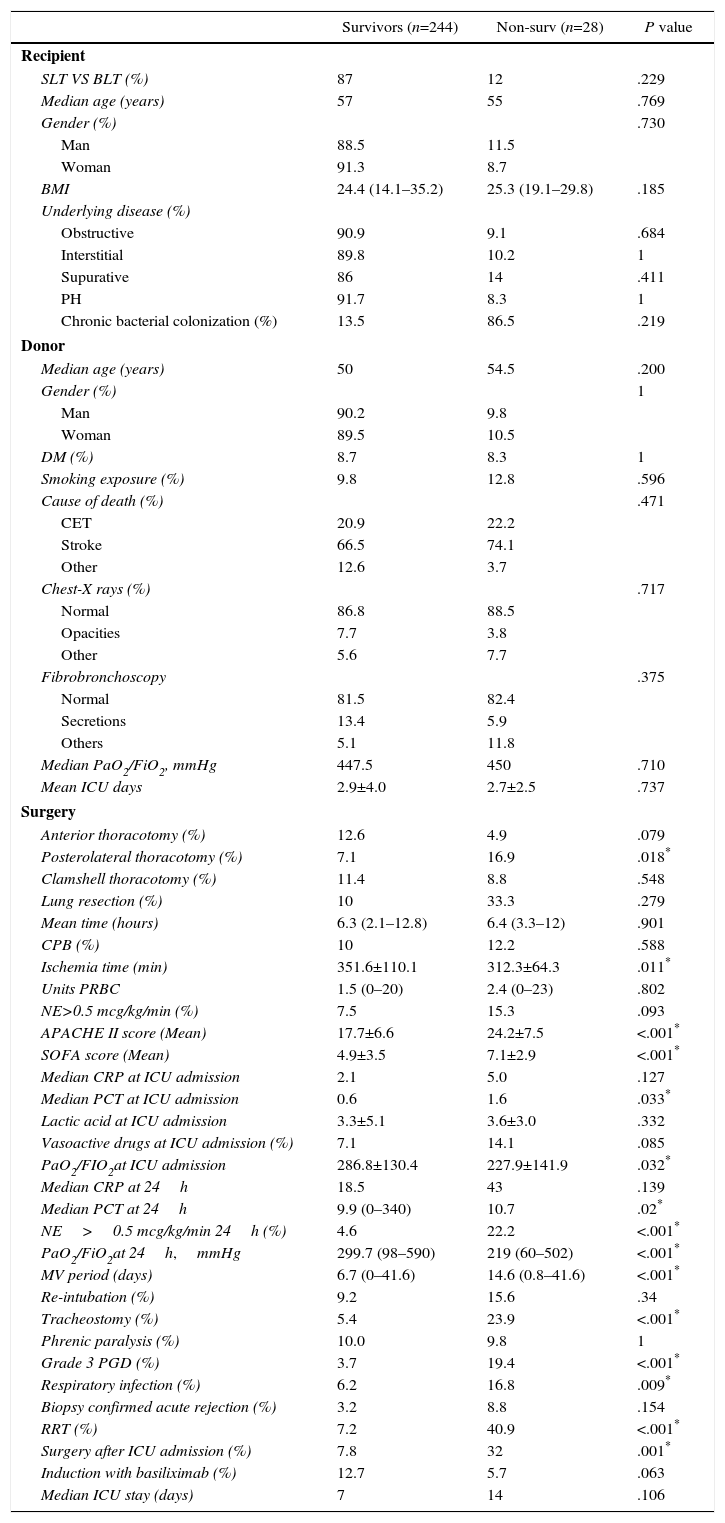
Median APACHE II score (24 vs 17) at ICU admission was significantly higher in non-survivors (OR: 1.12, 95%CI 1.06–1.19), as was median SOFA score (7 vs 5); OR: 1.14, 95%CI 1.03–1.29). The univariate analysis found that norepinephrine (>0.5mcg/kg/min) support after the first 24h had an OR of mortality of 5.87 (95%CI: 2.4–14.37). Grade 3 PGD was documented in 108 patients (39.9%), 21 of whom died, compared with 6of 163 without grade 3 PGD (P<.001). Thirteen were supported by extracorporeal membrane oxygenation and 7(53.8%) died. An association between days on mechanical ventilation and death was documented (OR: 1.04 per day; 95%CI 1.02 vs 1.07). Sixty-seven patients (24.8%) underwent tracheostomy. No significant differences were documented between mortality and diaphragmatic paralysis (9.8% vs 10%). Re-intubation was more frequent in patients who died (15.6% vs 9.2%, P<.05), and was reported in 18.8% of the cohort. Acute rejection therapy was started in 24 (16.6%) patients, being confirmed in half of them by biopsy. Renal replacement therapy was used in only 22 patients (8.1%) but this cohort had a mortality rate of 40.9%, with an OR of mortality of 8.88 (95%CI 3.35–23.5). A summary of the univariate analyses of postoperative (90-d) mortality is detailed in Table 1.
Univariate Analysis of 90-day Mortality.
| Survivors (n=244) | Non-surv (n=28) | P value | |
|---|---|---|---|
| Recipient | |||
| SLT VS BLT (%) | 87 | 12 | .229 |
| Median age (years) | 57 | 55 | .769 |
| Gender (%) | .730 | ||
| Man | 88.5 | 11.5 | |
| Woman | 91.3 | 8.7 | |
| BMI | 24.4 (14.1–35.2) | 25.3 (19.1–29.8) | .185 |
| Underlying disease (%) | |||
| Obstructive | 90.9 | 9.1 | .684 |
| Interstitial | 89.8 | 10.2 | 1 |
| Supurative | 86 | 14 | .411 |
| PH | 91.7 | 8.3 | 1 |
| Chronic bacterial colonization (%) | 13.5 | 86.5 | .219 |
| Donor | |||
| Median age (years) | 50 | 54.5 | .200 |
| Gender (%) | 1 | ||
| Man | 90.2 | 9.8 | |
| Woman | 89.5 | 10.5 | |
| DM (%) | 8.7 | 8.3 | 1 |
| Smoking exposure (%) | 9.8 | 12.8 | .596 |
| Cause of death (%) | .471 | ||
| CET | 20.9 | 22.2 | |
| Stroke | 66.5 | 74.1 | |
| Other | 12.6 | 3.7 | |
| Chest-X rays (%) | .717 | ||
| Normal | 86.8 | 88.5 | |
| Opacities | 7.7 | 3.8 | |
| Other | 5.6 | 7.7 | |
| Fibrobronchoscopy | .375 | ||
| Normal | 81.5 | 82.4 | |
| Secretions | 13.4 | 5.9 | |
| Others | 5.1 | 11.8 | |
| Median PaO2/FiO2, mmHg | 447.5 | 450 | .710 |
| Mean ICU days | 2.9±4.0 | 2.7±2.5 | .737 |
| Surgery | |||
| Anterior thoracotomy (%) | 12.6 | 4.9 | .079 |
| Posterolateral thoracotomy (%) | 7.1 | 16.9 | .018* |
| Clamshell thoracotomy (%) | 11.4 | 8.8 | .548 |
| Lung resection (%) | 10 | 33.3 | .279 |
| Mean time (hours) | 6.3 (2.1–12.8) | 6.4 (3.3–12) | .901 |
| CPB (%) | 10 | 12.2 | .588 |
| Ischemia time (min) | 351.6±110.1 | 312.3±64.3 | .011* |
| Units PRBC | 1.5 (0–20) | 2.4 (0–23) | .802 |
| NE>0.5 mcg/kg/min (%) | 7.5 | 15.3 | .093 |
| APACHE II score (Mean) | 17.7±6.6 | 24.2±7.5 | <.001* |
| SOFA score (Mean) | 4.9±3.5 | 7.1±2.9 | <.001* |
| Median CRP at ICU admission | 2.1 | 5.0 | .127 |
| Median PCT at ICU admission | 0.6 | 1.6 | .033* |
| Lactic acid at ICU admission | 3.3±5.1 | 3.6±3.0 | .332 |
| Vasoactive drugs at ICU admission (%) | 7.1 | 14.1 | .085 |
| PaO2/FIO2at ICU admission | 286.8±130.4 | 227.9±141.9 | .032* |
| Median CRP at 24h | 18.5 | 43 | .139 |
| Median PCT at 24h | 9.9 (0–340) | 10.7 | .02* |
| NE>0.5 mcg/kg/min 24h (%) | 4.6 | 22.2 | <.001* |
| PaO2/FiO2at 24h,mmHg | 299.7 (98–590) | 219 (60–502) | <.001* |
| MV period (days) | 6.7 (0–41.6) | 14.6 (0.8–41.6) | <.001* |
| Re-intubation (%) | 9.2 | 15.6 | .34 |
| Tracheostomy (%) | 5.4 | 23.9 | <.001* |
| Phrenic paralysis (%) | 10.0 | 9.8 | 1 |
| Grade 3 PGD (%) | 3.7 | 19.4 | <.001* |
| Respiratory infection (%) | 6.2 | 16.8 | .009* |
| Biopsy confirmed acute rejection (%) | 3.2 | 8.8 | .154 |
| RRT (%) | 7.2 | 40.9 | <.001* |
| Surgery after ICU admission (%) | 7.8 | 32 | .001* |
| Induction with basiliximab (%) | 12.7 | 5.7 | .063 |
| Median ICU stay (days) | 7 | 14 | .106 |
BLT: bilateral lung transplant; BMI: body mass index; CBP: cardiopulmonary bypass; CET: craneoencephalic trauma; CRP: C-reactive protein; DM: diabetes mellitus; PCT: procalcitonin hormone; PGD: primary graft dysfunction; PH: pulmonary hypertension; PRBC: packed red blood cells; MV: mechanical ventilation; NE: norepinephrine; RRT: renal replacement therapy; SLT: single lung transplant.
Data shown as percentages, median or mean (range).
APACHE II score, SOFA score, recipient age and grade 3 PGD were assessed in a multivariate stepwise logistic regression analysis. The model identified grade 3 PGD (OR 7.18, 95%CI 2.49–26.05), SOFA>8 (OR 2.86, 95%CI 0.81–8.85), and age>60yr (OR: 2.64, 95%CI 0.94–7.16) as independent predictors of 90-day mortality.
The same variables were entered in a second logistic regression model, which identified grade 3 PGD (OR: 6.77; 95%CI 2.72–19.46), donor age>60yr (OR: 2.91; 95%CI 7.07–1.18) and SOFA>8 (OR 2.53, 95%CI 0.80–7.26) as independent predictors of 28-day mortality.
At ICU admission, non-survivors had significantly lower (p=0.03) median PaO2/FiO2 (200mmHg vs 280mmHg); the difference increased after 24h (178 vs 297mmHg, P<.001). Using >300mmHg as a breakpoint, an OR of mortality of 0.30 (95%CI 0.12–0.78) was estimated. In contrast, C-reactive protein (CRP) (OR: 1.00; 95%CI 0.99–1.01) and lactate at ICU admission (OR: 1.01; 95%CI 0.90–1.08) did not show any association with mortality. However, non-survivors had significantly higher (p=0.03) median PCT (1.6 vs 0.6) at ICU admission, with the difference increasing after 24h (5.4 vs 1.8, P=.020). Using PCT>2.0 as the cut-off point, an OR of mortality of 2.05 (95%CI 0.79–5.34) was estimated.
DiscussionTo the best of our knowledge, this is the first multicenter study to assess prognostic factors in the early postoperative period following lung transplant in Spain. In this large cohort, we demonstrated an independent association between PGD, donor age above 60-yr or SOFA score >8 with early mortality after lung transplant. Non-survivors had higher hypoxemia and higher PCT levels (in contrast to lactate or CRP) at ICU admission, identifying patients at high risk of death.
The univariate analysis indicated an increased risk of death with the use of posterolateral thoracotomy, which is a surrogate of single lung transplant. PGD, which is a risk factor for mortality, has been associated with SLT.8 In contrast, other studies did not report a statistical association between PGD and SLT.9–12 Our findings are in line with the ISHLT registry,1 which found that SLT had worse survival than BLT. Our univariate analyses also showed that recipients with high APACHE II score and SOFA score >8 had a higher risk of mortality at 90 days. Oliveira et al.13 found that neither score had a better than average ability to predict hospital mortality in lung transplant recipients, and did not support their use in critically ill transplant patients. Arango et al.14 reported that although higher APACHE II scores were associated with prolonged mechanical ventilation and tracheotomy requirement, there was no association with higher rates of mortality or poor graft function. In our multivariate analysis, SOFA score >8 was associated with a 14% higher 90-day mortality, and in contrast to the APACHE II score, remained independently associated with death.
Graft failure is the main cause of death within the first 30 days after lung transplant and the second cause in the first year.1 In our cohort, grade 3 PGD was present in over one-third of the recipients. We found grade 3 PGD to be a strong independent risk factor of mortality in ICU (OR 6.32) and the only independent variable that was susceptible to alteration by intervention measures. This finding is consistent with previous reports15 of a relationship between severe PGD and early mortality. In our cohort, grade 3 PGD at 72h remained an important factor for mortality (OR 6.48; 95%CI 1.47–38.44).
Our results have implications for clinical practice and further research. Our findings in lung transplant are consistent with previous studies in other organ transplants,6,7,13 and support the use of the SOFA score over the APACHE II score. Excepting age, other donor variables did not influence outcomes. Indeed, grade 3 PGD was identified as the main variable associated with death, and our findings suggest that efforts should be targeted at preventing its development. Early ICU interventions in patients with severe hypoxemia or high PCT (rather than lactate or CRP) are crucial in order to reduce mortality. In lung transplant, biomarkers may require a different cut-off point than for sepsis, due to surgical injury. The optimal cut-off point for biomarkers was estimated based on Younder index values.16 In our cohort, PCT above 2 was associated with a two-fold increase in the risk of death. Some early biomarkers (oxygenation and PCT) helped identify differences in risk of death within 24h of surgery. This represents an opportunity for early identification of high-risk patients. Improved initial treatment in the ICU population may have an impact on outcome, particularly in the subset with grade 3 PGD. Further research into the identification of newer biomarkers, intra-operative biomarkers and postoperative breakpoints for Grade 3 PGF and mortality is now recommended.17,18
While our study is multi-center and does not suffer from the impact of secular trends (all patients from a single year, 2013), other limitations should be mentioned. For instance, we cannot assess data such as biomarkers for all patients, exposing the study to a potential selection bias. Indeed, bias inherent to the retrospective design were the main limitations of the study. Each center has different protocols, case-mix, experience, number of transplants, and resources, being associated with high variability. This problem is exacerbated by patient heterogeneity, non-protocolized management (e.g., criteria for biopsy, use of ECMO, empiric pulses of steroids) and variable immunosuppression regimens or surgical approach, and this compromises the robustness of the analysis. Some variables were significantly associated with a low number of deaths, and there may have been a type II statistical error. Absolute mortality at 90 days is 27 patients; although the denominator is substantial (and multicenter), this means that all of the (many) analyses relate to only 27 patients. Larger sample sizes would identify additional prognostic factors which remain hidden in our study. In addition, 2 key risk factors (age, admission hypoxemia) are not necessarily amenable to meaningful intervention. The strengths of this study are that the cohort included all patients who underwent lung transplant within 1 year in Spain, and that patients were followed-up for 90 days after ICU discharge. Early measure of procalcitonin was able to identify patients requiring better management due to high risk of death.
ConclusionsOur findings indicate that Grade 3 PGD is the main variable associated with death. The use of early biomarkers within 24h of surgery, such as oxygenation and PCT, can help differentiate patients in terms of risk of death. Thus, improved initial management in the ICU population may have an impact on outcome, particularly in the subset with grade 3 PGD.
AuthorshipThe study was designed by JCR and JRe. AHA, ELL, OG, JR, LR, RV, MB, EM, MS, MTRR, PRR and JCR reported patients. JCR coordinated and managed the database. JRe and IB wrote the first draft. The remaining authors revised the manuscript and approved the final version
Conflicts of InterestJR has received funding for travel or speaker's fees, and has served on scientific advisory boards for Pfizer Inc, Roche, Merck, Gilead Sciences, TermoFisher and Astellas Pharma Inc. The other authors have no conflicts of interest to declare.
FundingThe study was funded in part by CIBERES (InstitutoSalud Carlos III; Madrid), FISS 14/0114 and by an unrestricted research grant (PLUTO) from Astellas.
We would like to thank Luis Miguel Molinero Casares (AlceIngenieria, Valencia) for conducting the statistical analyses, and Michael Maudsley for help with the English.
Ana Hermira-Anchuelo, Elisabeth Coll, Eloísa López-Lopez, Olga González-González, Teresa Pont, Cristofer Mazo, Jordi Riera, Irene Bello, Luzdivina Rellan, Rosario de Vicente, Ma Angeles Ballesteros, Eduardo Miñambres, Margarita Sanchez, María Teresa Rey-Rilo. Pablo Rama-Maceiras, Juan Carlos Robles, Jordi Rello.