For many years now, the International Committee of Medical Journal Editors (ICMJE) has regularly published “Recommendations for the conduct, reporting, editing, and publication of scholarly work in medical journals”, setting standards for many medical journals and from other disciplines.1 Several thousand journals that currently adhere to these Recommendations appear on the list published on the ICMJE website. However, the fact that a journal appears on this list does not presuppose any obligation to comply with the Recommendations.2,3
Some time ago, Archivos de Bronconeumología reported on a radical turnabout by the ICMJE: after announcing in 2016 that they would require clinical trial researchers to share individual-level anonymized participant data with third parties, in 2017 they decided that such transfer would be voluntary.4 The news had a precedent in the Recommendations published a few years earlier, to the effect that some journal editors “ask authors to say whether the study data are available to third parties to view and/or use/reanalyze, while still others encourage or require authors to share their data with others for review or reanalysis”.1 It would be interesting to know which Spanish journals have included this requirement in their ‘instructions for authors’ and whether they comply with it.
To answer this question, we reviewed the portals of 24 Spanish journals with an impact factor greater than 1, on the understanding that they have greater influence than those with an impact factor ≤1 and those with no impact factor. Of these 24, 14 are included in the list of ICMJE Recommendations (Supplementary material A). Of these, only 5 (Archivos of Bronconeumología, Atención Primaria, Enfermedades Infecciosas y Microbiología Clínica, Gaceta Sanitaria, and Medicina Intensiva) include a specific section, that we shall call “link to data repository”, that recommends, supports and encourages authors to share raw data from their studies with other researchers, and gives instructions on how to go about it. A sixth journal, the Revista de Neurología, recommends this procedure only for clinical trials (Supplementary material B). To determine the frequency with which authors report how data can be accessed compared to other requirements requested by the same journals, 2 control requirements were selected: reporting on conflicts of interest and study funding, that were included in the Recommendations much earlier. It is also of interest to determine whether supplementary material may be included online, as this is sometimes a way of including raw study data.
The first 25 articles published in each of the 6 journals since the first issue of 2022 were reviewed. Studies of any design that were more readily applicable to human health were included in the analysis, i.e., those carried out in humans, on their data, opinions, or samples. In vitro studies, animal studies, mathematical models, and reports on 5 cases or fewer were excluded. For all the studies, the 4 requirements mentioned above were recorded, along with the type of article, and the country where the first author conducted the work. To determine if a particular journal that, despite not requiring a specific ‘link to data repository’, included this information in their articles, the first 25 articles from 2022 of the 3 journals included in the list of Recommendations with the highest impact factor were reviewed: Revista Española de Cardiología, Journal of Investigational Allergology & Clinical Immunology and Medicina Clínica.
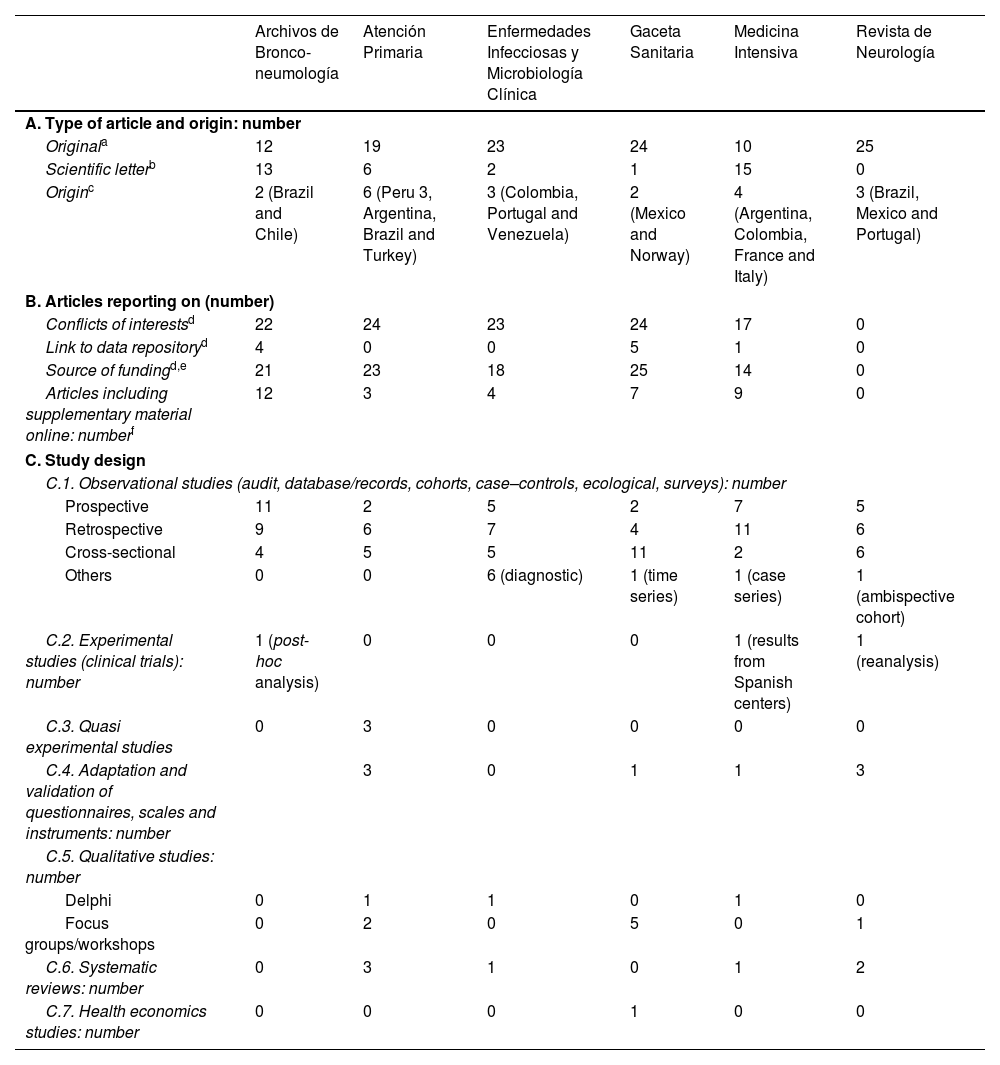
The results show (Table 1) that (a) of the 150 articles studied in the 6 journals that were analyzed, only 10 (7%) – from 3 journals – include a section on ‘link to data repository’; (b) the Revista de Neurología does not report any of the variables analyzed; (c) both conflicts of interest and sources of funding are frequently reported, the former slightly more so, except in Medicina Intensiva; (d) a minority of articles are accompanied by supplementary material; and (e) the variety of disciplines covered by the journals analyzed meant that all kinds of studies were published, although most (78%, 117/150) were observational, and only 3 articles referred to clinical trials. In the 75 articles reviewed from the 3 control journals, none included a section on ‘link to data repository’. The Revista Española de Cardiología and the Journal of Investigational Allergology & Clinical Immunology had a very high percentage of compliance with regard to the declaration of conflicts of interest and sources of funding (Supplementary material C).
Type of Articles, Number of Articles Reporting the Variables Analyzed and the Design of the Studies Included in the 150 Articles Analyzed From the 6 Spanish Journals Studied (the First 25 Articles From Each Journal Published in 2022).
| Archivos de Bronco-neumología | Atención Primaria | Enfermedades Infecciosas y Microbiología Clínica | Gaceta Sanitaria | Medicina Intensiva | Revista de Neurología | |
|---|---|---|---|---|---|---|
| A. Type of article and origin: number | ||||||
| Originala | 12 | 19 | 23 | 24 | 10 | 25 |
| Scientific letterb | 13 | 6 | 2 | 1 | 15 | 0 |
| Originc | 2 (Brazil and Chile) | 6 (Peru 3, Argentina, Brazil and Turkey) | 3 (Colombia, Portugal and Venezuela) | 2 (Mexico and Norway) | 4 (Argentina, Colombia, France and Italy) | 3 (Brazil, Mexico and Portugal) |
| B. Articles reporting on (number) | ||||||
| Conflicts of interestsd | 22 | 24 | 23 | 24 | 17 | 0 |
| Link to data repositoryd | 4 | 0 | 0 | 5 | 1 | 0 |
| Source of fundingd,e | 21 | 23 | 18 | 25 | 14 | 0 |
| Articles including supplementary material online: numberf | 12 | 3 | 4 | 7 | 9 | 0 |
| C. Study design | ||||||
| C.1. Observational studies (audit, database/records, cohorts, case–controls, ecological, surveys): number | ||||||
| Prospective | 11 | 2 | 5 | 2 | 7 | 5 |
| Retrospective | 9 | 6 | 7 | 4 | 11 | 6 |
| Cross-sectional | 4 | 5 | 5 | 11 | 2 | 6 |
| Others | 0 | 0 | 6 (diagnostic) | 1 (time series) | 1 (case series) | 1 (ambispective cohort) |
| C.2. Experimental studies (clinical trials): number | 1 (post-hoc analysis) | 0 | 0 | 0 | 1 (results from Spanish centers) | 1 (reanalysis) |
| C.3. Quasi experimental studies | 0 | 3 | 0 | 0 | 0 | 0 |
| C.4. Adaptation and validation of questionnaires, scales and instruments: number | 3 | 0 | 1 | 1 | 3 | |
| C.5. Qualitative studies: number | ||||||
| Delphi | 0 | 1 | 1 | 0 | 1 | 0 |
| Focus groups/workshops | 0 | 2 | 0 | 5 | 0 | 1 |
| C.6. Systematic reviews: number | 0 | 3 | 1 | 0 | 1 | 2 |
| C.7. Health economics studies: number | 0 | 0 | 0 | 1 | 0 | 0 |
(a) Includes brief report; (b) includes 3 ‘letters’ reporting on study results; (c) only the foreign country where the first author worked is indicated. (d) The 6 journals state that authors must publish the link to the data repository, so that other researchers can use them, and report on conflicts of interest and sources of funding; (e) the statement appears, but without taking into account when funding only allowed the publication of the article in open-access; (f) all, except Revista de Neurología, allow additional material to be included online.
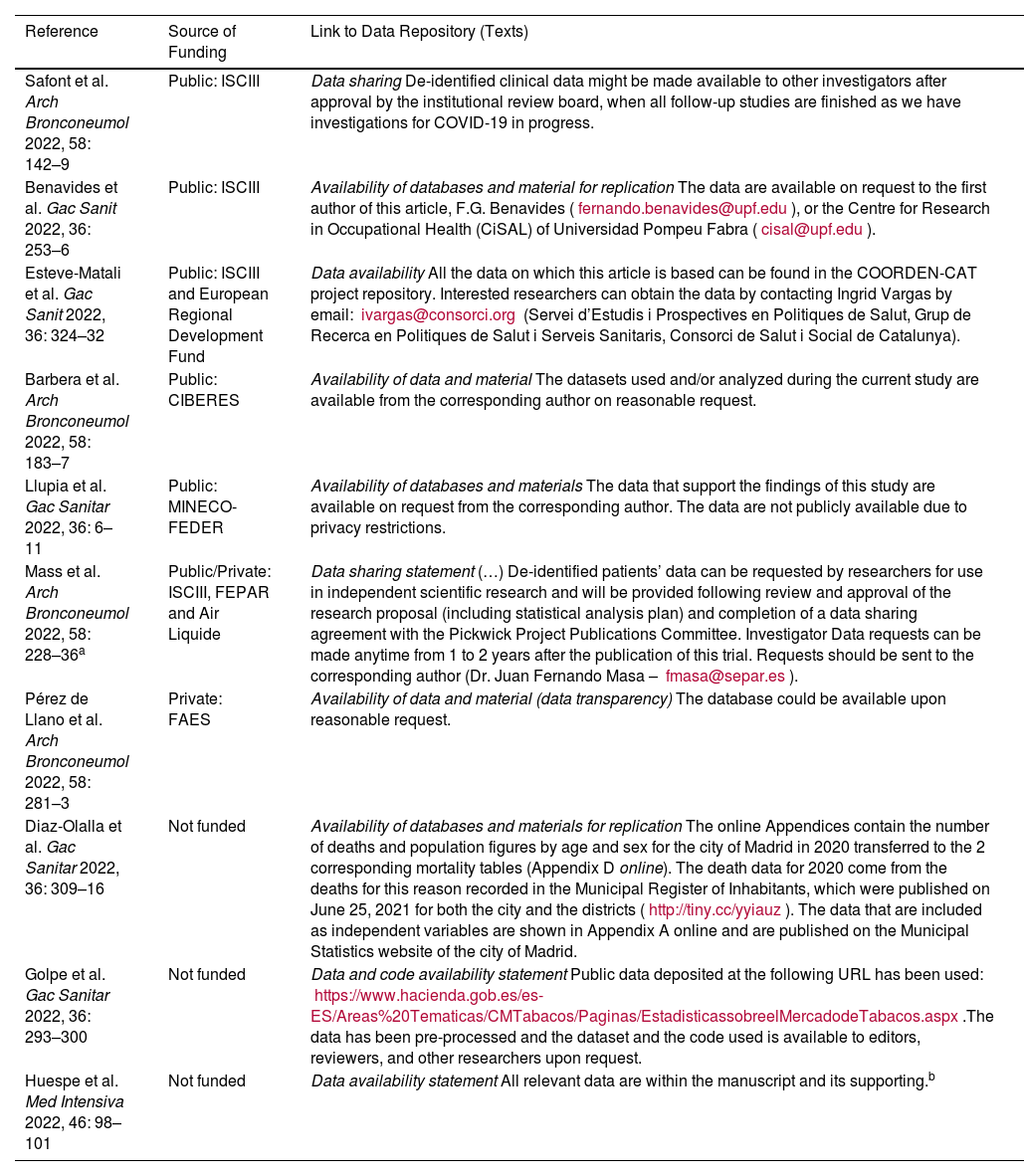
This analysis showed that very few prestigious Spanish medical journals ask authors to make their raw study data available to third parties. More worrying is the fact that among the few who request it, some have recently published up to 25 articles without a single one having made their data available to other researchers. Furthermore, among the journals that recommend doing so, only a minority of articles inform interested readers how to access these data. As can be seen from the examples shown in Table 2, there are many ways to report on data sharing, yet in only one case (Table 2 – Huespe et al.) the authors stated that the data was included in the supplementary material… which was not included in the article. Note that the examples of articles that do report readers how to access the scientific data of the study mention sources of public and private funding, and some are even unfunded. The fact that the study has received public funding (e.g., FEDER, regional governments) does not appear to compel researchers to share the data, since some of the reviewed articles did not report interested readers how to access them.
Articles Including Information on How to Share Raw Study Data With Third Parties.
| Reference | Source of Funding | Link to Data Repository (Texts) |
|---|---|---|
| Safont et al. Arch Bronconeumol 2022, 58: 142–9 | Public: ISCIII | Data sharing De-identified clinical data might be made available to other investigators after approval by the institutional review board, when all follow-up studies are finished as we have investigations for COVID-19 in progress. |
| Benavides et al. Gac Sanit 2022, 36: 253–6 | Public: ISCIII | Availability of databases and material for replication The data are available on request to the first author of this article, F.G. Benavides (fernando.benavides@upf.edu), or the Centre for Research in Occupational Health (CiSAL) of Universidad Pompeu Fabra (cisal@upf.edu). |
| Esteve-Matali et al. Gac Sanit 2022, 36: 324–32 | Public: ISCIII and European Regional Development Fund | Data availability All the data on which this article is based can be found in the COORDEN-CAT project repository. Interested researchers can obtain the data by contacting Ingrid Vargas by email: ivargas@consorci.org (Servei d’Estudis i Prospectives en Politiques de Salut, Grup de Recerca en Politiques de Salut i Serveis Sanitaris, Consorci de Salut i Social de Catalunya). |
| Barbera et al. Arch Bronconeumol 2022, 58: 183–7 | Public: CIBERES | Availability of data and material The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. |
| Llupia et al. Gac Sanitar 2022, 36: 6–11 | Public: MINECO-FEDER | Availability of databases and materials The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions. |
| Mass et al. Arch Bronconeumol 2022, 58: 228–36a | Public/Private: ISCIII, FEPAR and Air Liquide | Data sharing statement (…) De-identified patients’ data can be requested by researchers for use in independent scientific research and will be provided following review and approval of the research proposal (including statistical analysis plan) and completion of a data sharing agreement with the Pickwick Project Publications Committee. Investigator Data requests can be made anytime from 1 to 2 years after the publication of this trial. Requests should be sent to the corresponding author (Dr. Juan Fernando Masa – fmasa@separ.es). |
| Pérez de Llano et al. Arch Bronconeumol 2022, 58: 281–3 | Private: FAES | Availability of data and material (data transparency) The database could be available upon reasonable request. |
| Diaz-Olalla et al. Gac Sanitar 2022, 36: 309–16 | Not funded | Availability of databases and materials for replication The online Appendices contain the number of deaths and population figures by age and sex for the city of Madrid in 2020 transferred to the 2 corresponding mortality tables (Appendix D online). The death data for 2020 come from the deaths for this reason recorded in the Municipal Register of Inhabitants, which were published on June 25, 2021 for both the city and the districts (http://tiny.cc/yyiauz). The data that are included as independent variables are shown in Appendix A online and are published on the Municipal Statistics website of the city of Madrid. |
| Golpe et al. Gac Sanitar 2022, 36: 293–300 | Not funded | Data and code availability statement Public data deposited at the following URL has been used: https://www.hacienda.gob.es/es-ES/Areas%20Tematicas/CMTabacos/Paginas/EstadisticassobreelMercadodeTabacos.aspx.The data has been pre-processed and the dataset and the code used is available to editors, reviewers, and other researchers upon request. |
| Huespe et al. Med Intensiva 2022, 46: 98–101 | Not funded | Data availability statement All relevant data are within the manuscript and its supporting.b |
(a) This article reports the results of a post-hoc analysis of a clinical trial; (b) please note that the sentence is incomplete. Furthermore, this study was not accompanied by any supplementary material, as the sentence suggests. Thus, the interested reader cannot access the information that the authors are supposed to make available. This is a study conducted in Argentina and the only one by foreign authors included in this table.
CIBERES: Biomedical Research Center in Respiratory Diseases Network; FAES: a Spanish pharmaceutical company; FEDER: European Union Funds; ISCIII: Instituto de Salud Carlos III; MINECO: Ministry of Economic Affairs and Digital Transformation.
The greatest limitation of this analysis lies in the small number of journals that make up the study sample. To perform a more comprehensive analysis, journals with an impact factor of less than 1 would also have to be included, and perhaps even those that have not yet been assigned an impact factor. Although this analysis has included a very limited number of articles, it is not unreasonable to suggest that journals should make an effort to ensure that the articles they publish fulfil their own requirements. If journals such as the Journal of Investigational Allergology & Clinical Immunology and Gaceta Sanitaria manage to publish articles that comply with conflicts of interest statements (100% and 98%, respectively), we wonder why the same is not true of the ‘link to data repository’. At the opposite extreme, the situation of Revista de Neurología is surprising and may be explained by the decision of the editorial team to report none of the requirements analyzed in this study.
Sharing raw data promotes medical knowledge.5 Sharing data from quantitative studies is much easier than from qualitative studies. Researchers performing qualitative studies frequently cite the lack of authorization of the participants, the sensitive nature of the data, and loss of confidentiality as reasons for not sharing data.6 However, qualitative studies are the exception among Spanish medical publications. By 2011, most researchers were already sharing their data, although this was challenging for more than a third of them; in the case of clinical trials, it has recently been reported that access7 to data is difficult despite authors’ commitment to share.8 Ideally, Spanish medical journals should require authors to share them in all the articles they publish, and if data sharing is impossible, to explain why.
Conflicts of InterestsThe author states that he has no conflict of interests.
Availability of data and materialAll raw data are provided in the supplemantary material.