Although during the last few years there have been several important changes in the diagnostic or therapeutic methods, pleural effusion is still one of the diseases that the respiratory specialist have to evaluate frequently. The aim of this paper is to update the knowledge about pleural effusions, rather than to review the causes of pleural diseases exhaustively. These recommendations have a longer extension for the subjects with a direct clinical usefulness, but a slight update of other pleural diseases has been also included. Among the main scientific advantages are included the thoracic ultrasonography, the intrapleural fibrinolytics, the pleurodesis agents, or the new pleural drainages techniques.
A pesar de los múltiples avances diagnósticos o terapéuticos de la medicina de los últimos años, el derrame pleural (DP) continúa siendo una de las enfermedades que con frecuencia tiene que abordar el especialista de aparato respiratorio o el cirujano torácico. El presente texto no tiene como objetivo realizar una revisión exhaustiva sobre las enfermedades que pueden producir DP, su diagnóstico o su tratamiento, sino constituir una actualización de los conocimientos publicados en los últimos años. Teniendo en cuenta la vocación eminentemente práctica de esta normativa, se ha concedido más extensión a las enfermedades que presentan una mayor incidencia o prevalencia, aunque no hemos renunciado a un ligero recordatorio de otras menos frecuentes. Entre los mayores avances destacan los conocimientos sobre la utilidad de la ecografía torácica, los fibrinolíticos y los agentes pleurodésicos, o la utilización de nuevas técnicas de drenaje pleural, como los tubos torácicos finos o los catéteres tunelizados. La actualización periódica de las normativas favorece la potencial incorporación de nuevas técnicas en el estudio de la enfermedad pleural.
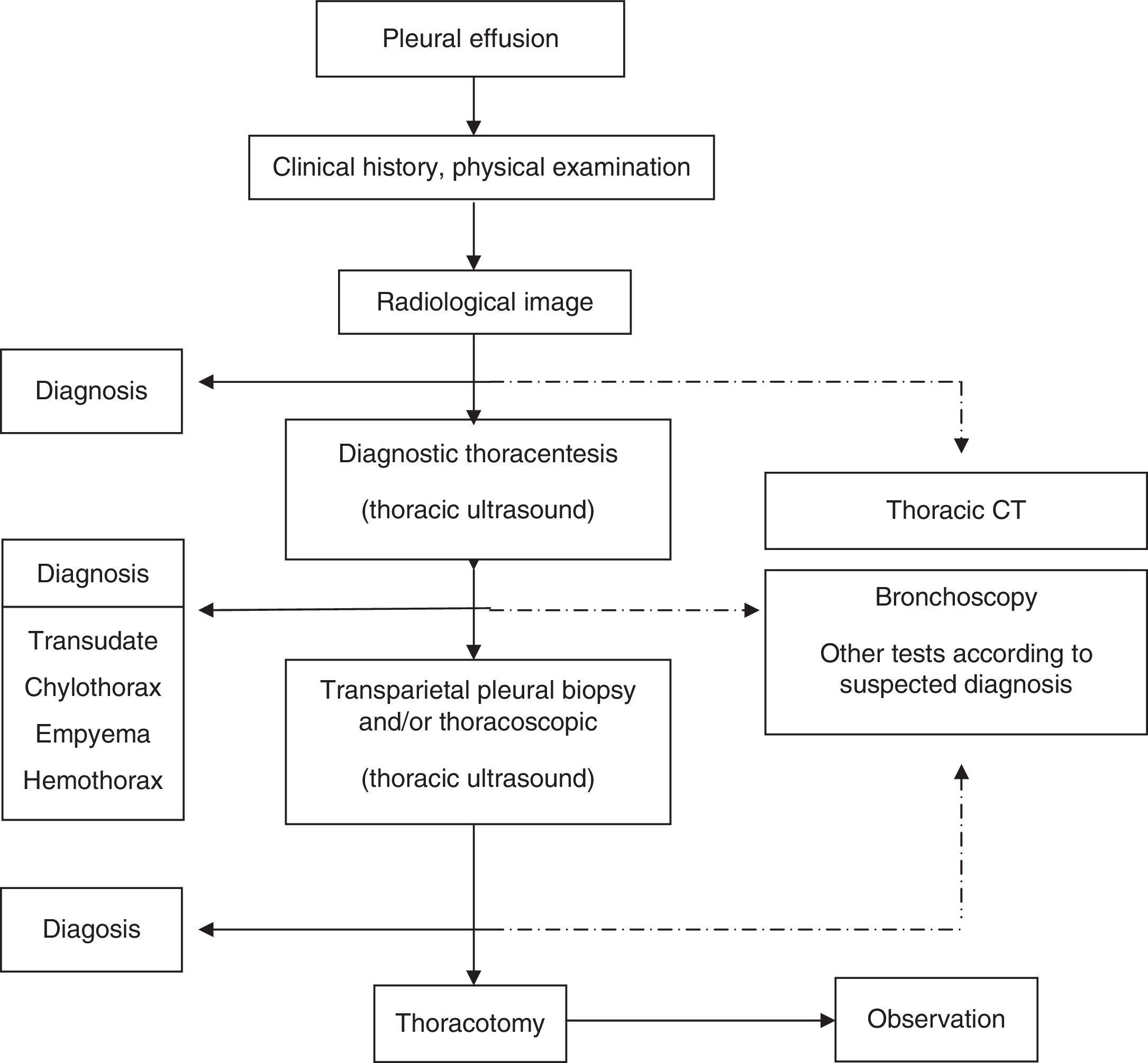
Complete history and physical examination should be performed, including an evaluation of disease, employment and medication history. The most usual imaging technique for identifying PE is posteroanterior chest X-ray.1 Thoracic ultrasound (US) should be easily accessible for these patients. It is also recommended that US be performed by the same physician who performs the puncture, in order to increase diagnostic yield and reduce the complications of thoracentesis (A).3 US is more sensitive than X-ray in identifying PE, and better than computed tomography (CT) for identifying septa (C). Its indications also include locating small or encapsulated PE for puncture or biopsy, characterization of the fluid or pleural surface, or providing guidance regarding the entry point for thoracoscopy. Its use is recommended for guidance in all pleural invasive techniques (B) and, if possible US should be performed immediately before the technique to avoid puncture in a previously marked entry point (F).4 Chest CT may be useful for modifying the probability of identifying malignancy in PE, for locating suitable areas for biopsy or for identifying other pathological regions, such as the lung parenchyma or the mediastinum. Abdominal CT may be useful for ruling out infradiaphragmatic pathologies causing PE.
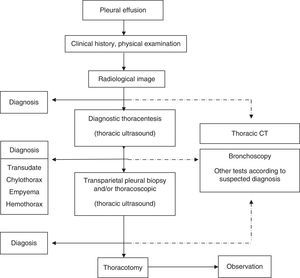
A presumptive diagnosis should be established from the clinical and radiological findings. The main causes of PE were specified in previous guidelines.1 Thoracentesis is not indicated for patients with bilateral PE if clinical signs suggest a strong suspicion of transudate (H).5 Thoracentesis will be performed in all other situations, the amount of fluid permitting. Sample preparation and the main findings in pleural fluid (PF) were specified in previous guidelines.1 If PF analysis is not sufficient to establish diagnosis, pleural tissue samples will be taken by transmural pleural biopsy (strong suspicion of tuberculosis and in experienced centers) or thoracoscopy. Image-guided pleural biopsy increases the sensitivity of biopsy to values close to those of thoracoscopy.6 Bronchoscopy is indicated in the presence of bronchial symptoms (hemoptysis, changes in cough or sputum), or if nodules or pulmonary masses or signs suggestive of bronchial obstruction are observed on radiological examination. A diagnostic schematic for patients with PE is shown in Fig. 1.
Differentiation of Transudative and Exudative Pleural EffusionDifferentiation between transudative and exudative effusion is considered the initial step in the etiological diagnosis of any PE. The former results from an imbalance between the hydrostatic and oncotic forces in the pulmonary or systemic circulation, whereas the latter is produced by increased vascular permeability. Transudates are most often caused by heart failure (80%) and, to a lesser extent, by hepatic cirrhosis. Additional diagnostic procedures are usually not required. Conversely, exudates require more extensive diagnostic evaluation, since they have numerous etiologies.1 Nevertheless, in 80% of cases, the exudate is secondary to cancer, pneumonia, tuberculosis or viral pleuropericarditis. In clinical practice, the difference between exudates and transudates is established with Light's criteria (B), according to which PE is exudate if it meets one or more of the following conditions:
- -
The ratio of pleural fluid protein to serum protein is greater than 0.5.
- -
The ratio of pleural fluid lactate dehydrogenase (LDH) to serum LDH is greater than 0.6.
- -
LDH content in PF is greater than 2/3 of the upper limit of normal serum levels of LDH.
Almost all exudates (98%) are correctly identified using these criteria, but approximately 30% of cardiac PEs and 20% of hepatic hydrothoraces are classified erroneously as exudates.7 This circumstance is particularly prevalent in patients receiving diuretic therapy or in the presence of bloody PEs. If heart failure is suspected, but the PE is a borderline exudate, calculation of the gradient (difference) between serum albumin levels and PF is recommended. If the difference is greater than 1.2g/dL, a circumstance occurring in 83% of patients with these cardiac “false exudates”, PE will be assumed to be transudative.7 Hepatic hydrothoraces labeled as exudates by Light's criteria show a ratio between PF and serum albumin lower than 0.6 in 77% of cases.7 If available, pleural concentrations of natriuretic peptide NT-proBNP above 1500pg/mL are very useful for the diagnosis of heart failure (positive probability ratio greater than 10),8 and correlate well with blood measurements of NT-proBNP. Other criteria with poorer yield have been proposed for exudate classification, such as cholesterol levels greater than 60mg/dL in PF, or a PF/serum cholesterol ratio greater than 0.3.
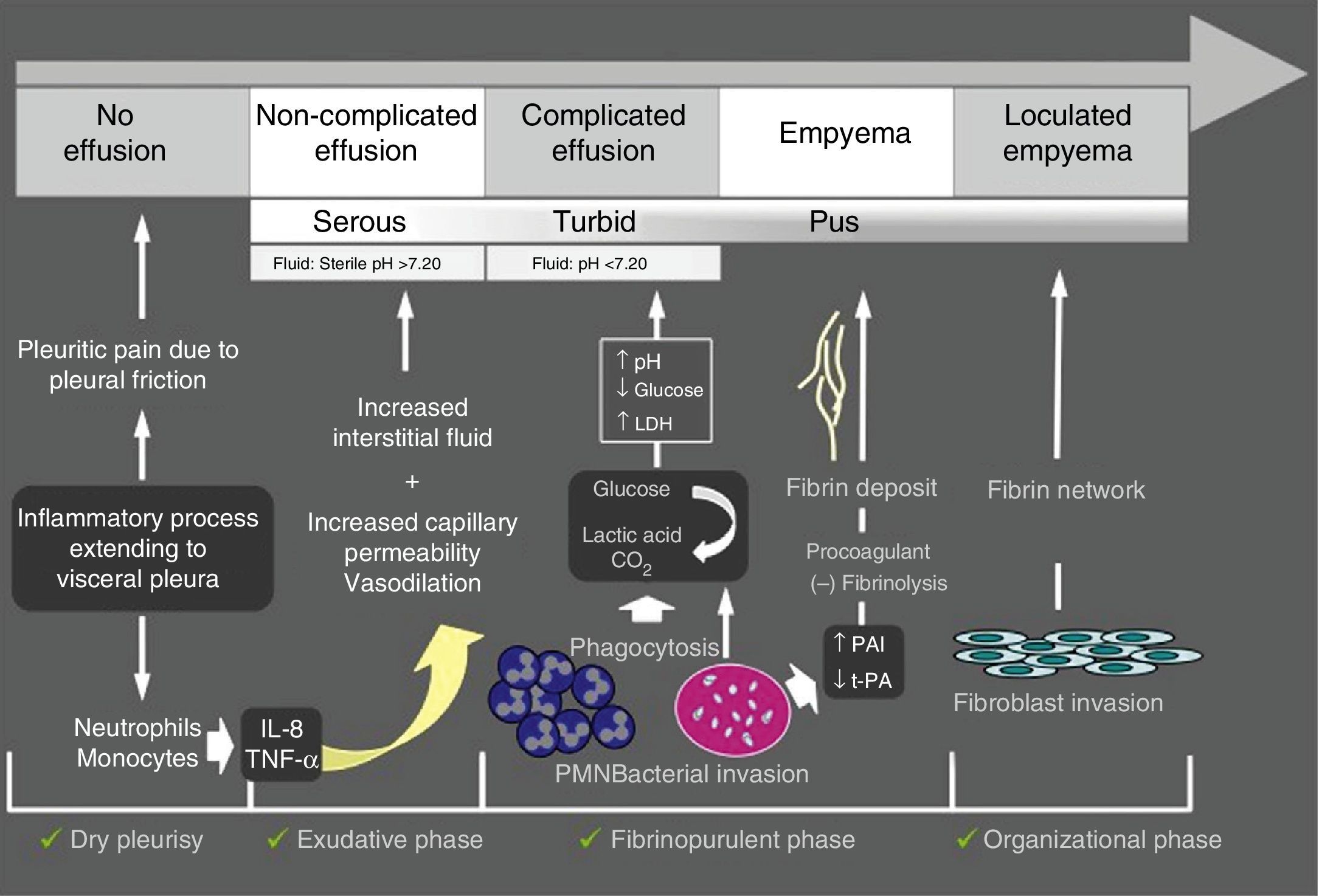
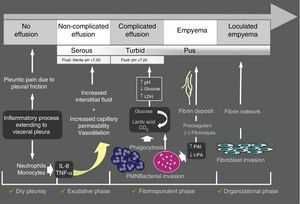
Parapneumonic Pleural EffusionParapneumonic pleural effusion (PPPE) is associated with pulmonary infection, usually pneumonia, abscess, or infected bronchiectasis. Between 20% and 57% of bacterial pneumonias are accompanied by PPPE during their clinical course and approximately 40% of these are complicated PPPE or empyema.9 Therefore, PPE should be considered in all patients with pneumonia. PPPE occurs most often at the beginning and the end of life, and two thirds of patients with complicated PPPE or empyema have an associated risk factor, such as lung disease (bronchiectasis, COPD, lung cancer, previous tuberculosis), systemic diseases favoring aspiration or immunodeficiency. Fig. 2 shows the pathogenesis of PPPE.
Incidence of microorganism isolation is highly variable and no responsible microorganism is found in over 40% of empyemas. In PPPEs associated with community-acquired pneumonia, the most frequently isolated Gram-positive bacteria are aerobic, such as streptococci (Streptococcus milleri and Streptococcus pneumoniae) and Staphylococcus aureus, followed by anaerobic microorganisms (common in aspiration pneumonia), and a small group of Gram-negative bacteria (Enterobacteriaceae, Escherichia coli and Haemophilus influenzae) in patients with comorbid conditions, especially diabetes or alcoholism.10 In nosocomial pneumonia, the most frequent pathogen is S. aureus, and 60% correspond to methicillin-resistant S. aureus. The next most frequent are gram-negative aerobes (E. coli, Enterobacter spp. and Pseudomonas spp.), along with anaerobes. Fungal infections are rare, and Candida spp. is the most common fungus, especially in immunocompromised patients.
DiagnosisDuring the evaluation of any pneumonia, the possibility of PPPE should be considered. Radiological studies are important (standard X-ray, CT, ultrasound, and thoracentesis). Contrast-enhanced CT is useful in differentiating lung consolidation, with contrast medium uptake, from a hypodense PE. Interlobular, mediastinal pleural and small-sized paravertebral collections are identified on CT. With CT, a peripheral abscess can be differentiated from complicated PPPE with the split pleura sign (thickened parietal and visceral pleura with displacement of surrounding vessels) that distinguishes it from a lung abscess. Air inside the PPPE, in the absence of previous maneuvers, may be due to the presence of gas-forming bacteria, or empyema progressing with a bronchopleural or pleuroparietal fistula (empyema necessitatis). Pleural thickening or attenuation of subcostal fat on CT suggest infection of the pleural cavity, and may also be useful for detecting malignancy associated with PPPE.11 However, CT has low sensitivity for displaying septa in the pleural cavity.
Thoracic ultrasound helps identify small PE. The presence of septa on ultrasonography suggests complicated PPPE and hyperechogenicity is associated with pus in the pleural cavity.12 Ultrasound provides guidance for the best location for drainage placement, improves its performance, and reduces the risk of complications (B).
Thoracentesis should always be performed when PE is suspected (B), and blood cultures should be extracted (B). Uncomplicated PPPE may become complicated in less than 12h, so close clinical management is required. Presence of microorganisms, turbidity or putrid odor confirms the diagnosis of PPPE, which is considered empyema when it contains pus. Frequently PF cultures are negative or an early therapeutic decision must be made before results are available (24–48h). In this case, pH is the best marker (A),13 but other biochemical parameters such as glucose or LDH levels are also of great diagnostic and prognostic use. It should be remembered that pH may vary in the different loculated PE chambers, and also that it is acid in malignant PE, rheumatoid arthritis, lupus pleuritis and tuberculous PE. Conversely, Proteus spp. causes PF alkalinization.
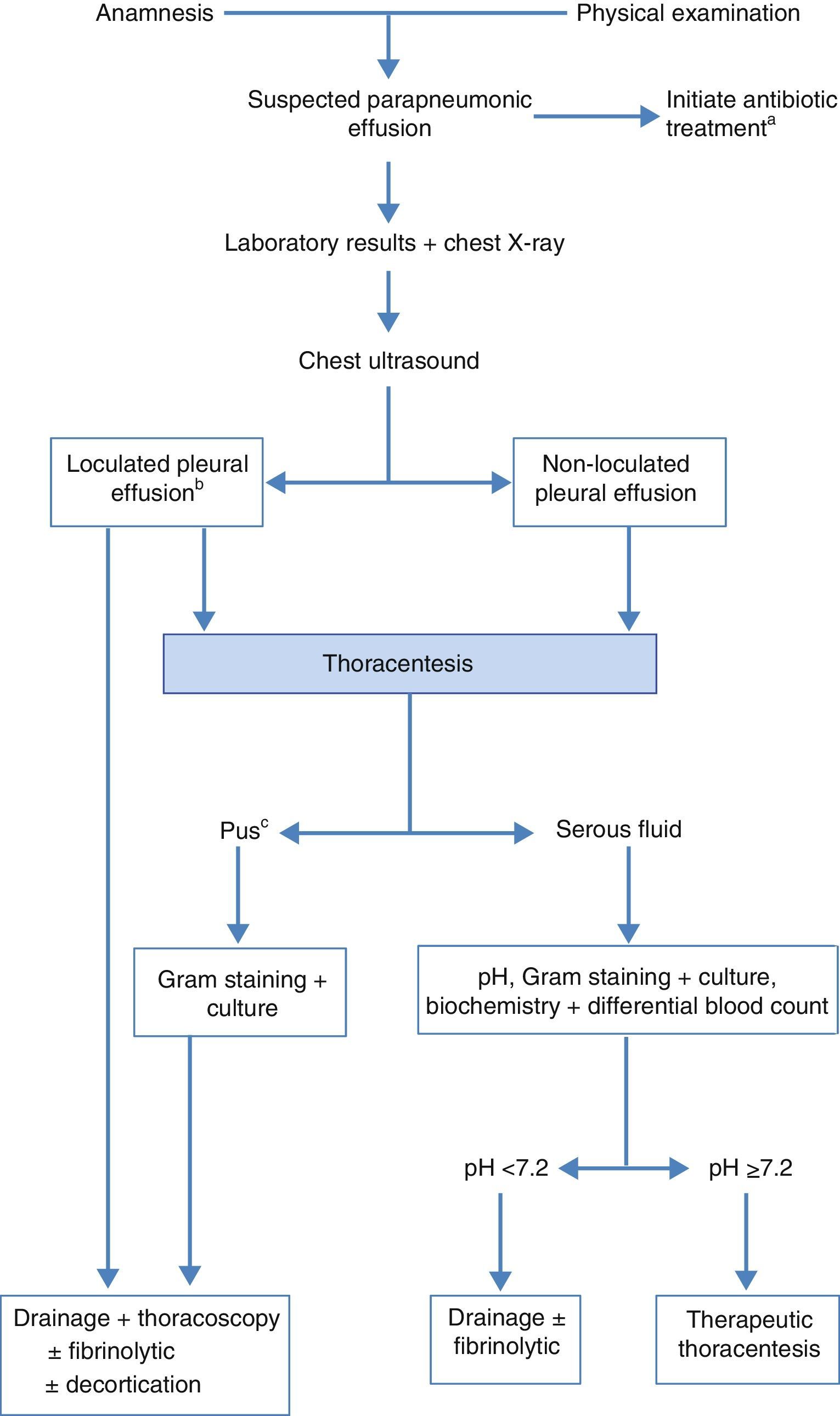
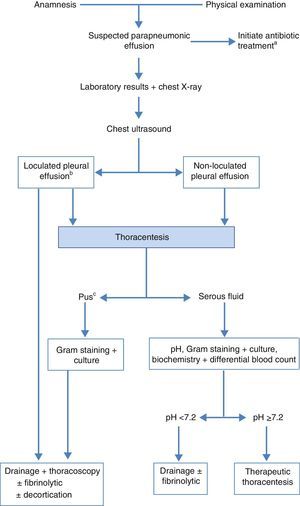
TreatmentThe objective is to control the infection with the appropriate antibiotic and drain the infected and/or complicated PE.14,15 Good nutrition, particularly in hypoproteic patients, and antithrombotic prophylaxis should not be forgotten.11Fig. 3 shows the algorithm for the treatment of PPPE.
Algorithm for the treatment of parapneumonic effusion. (a) early empirical antibiotic treatment should be initiated in all cases, and then adjusted with the culture results; (b) the presence of loculation can also be based on the findings of CT scan or chest X-ray; (c) also if the pleural fluid is turbid or smelly.
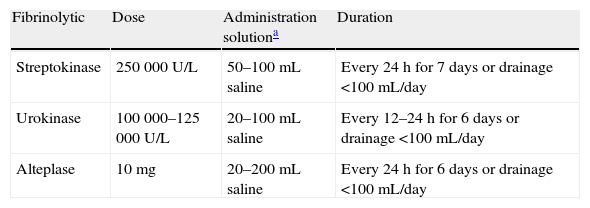
Early, empirical antibiotic treatment with anaerobic cover should be initiated (C), and adjusted after the cultures result are available. Antibiotic selection should take into account the community or nosocomial origin of the PPPE, patient characteristics, geographical and local microbiological peculiarities, and antibiotic activity in the PF (B). A combination of a third generation cephalosporin with clindamycin or metronidazole, or monotherapy with amoxicillin-clavulanate may be used. Patients with penicillin allergies may be treated with clindamycin, usually in combination with a quinolone. If the source is a nosocomial infection, recommended antibiotics include vancomycin or linezolid (for methicillin-resistant S. aureus), antipseudomonal penicillins (piperacillin-tazobactam), carbapenem, or third-generation cephalosporins with metronidazole. Importantly, aminoglycosides have poor pleural penetration and intrapleural antibiotics are not indicated (H). Therapeutic thoracentesis is an effective procedure for moderately sized PEs that do not meet criteria for chest tube drainage (H). Chest tube is indicated in all cases of empyema or complicated PPPE (pH less than 7.20, loculated PPPE or bacteria in the PF) (B). When pH determination is not available, glucose levels below 60mg/dL and LDH levels above 1000U/L are useful for identifying the need for pleural drainage.13 It should be pointed out that pleural drainage may be required if clinical progress is slow, even if pH is greater than 7.20. In loculated PE, chest ultrasound facilitates drainage positioning and placement, especially if more than one tube is needed to drain different cavities. There is no consensus on the most appropriate tube gauge for drainage, but small catheters (10–14 French) are easier to position, less traumatic and more comfortable for the patient. Additionally, if fibrinolytic therapy and lavage are also applied, efficacy is similar to that of tubes of greater gauge.16 Drainage is considered to have failed when sepsis persists despite antibiotic therapy. This may be due to incorrect drainage placement, the presence of multiple loculations, late initiation of antibiotic treatment, or presence of a bronchopleural fistula. Fibrinolytics should be started early in the case of loculations in the pleural cavity and empyema. These agents facilitate drainage of dense fluids and prevent the formation of septa in the pleural cavity. Streptokinase, urokinase or alteplase with DNase17 are most often used, but there is no consensus on the dose.18Table 1 summarizes the most used regimens. Sufficient scientific evidence to recommend a particular agent or regimen is not available.
Fibrinolytic Doses for Treating Pleural Parapneumonic Effusion.
| Fibrinolytic | Dose | Administration solutiona | Duration |
| Streptokinase | 250000U/L | 50–100mL saline | Every 24h for 7 days or drainage <100mL/day |
| Urokinase | 100000–125000U/L | 20–100mL saline | Every 12–24h for 6 days or drainage <100mL/day |
| Alteplase | 10mg | 20–200mL saline | Every 24h for 6 days or drainage <100mL/day |
Treatment with fibrinolytics is safe and with few side effects; it improves radiological progression and decreases the days of drainage and hospital stay.19 The only contraindication is bronchopleural fistula.
Early thoracoscopy is an option for patients with loculated PPPE. It allows pleural debridement with the subsequent lung reexpansion, pus evacuation and drainage placement. Surgical treatment is indicated for control of sepsis when medical treatment and drainage with fibrinolytics fail.
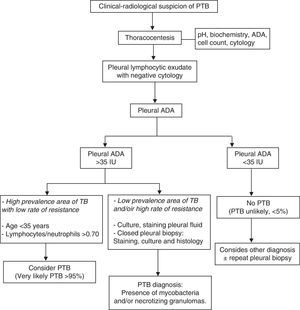
Tuberculous Pleural EffusionTuberculosis (TB) is a major public health problem, and its prevalence in Spain is 30/100000 inhabitants. Pleural tuberculosis (PTB) represents 4%–10% of all TB cases and 10%–15% of large series of PE confirmed by thoracentesis in Spain. PTB is usually the result of a compartmentalized immune response against a few antigenic components of M. tuberculosis that reach the pleura from subpleural foci. This response is mediated by mesothelial cells, neutrophils, Th1 lymphocytes (CD4), monocytes and related cytokines (IL1-6, IL-8, interferon-gamma (INFγ), and vascular endothelial growth factor [VEGF]), responsible for local inflammation, increased vascular permeability and the accumulation of fluid in the pleural space.20
In Spain, PTB particularly affects young people under 35 years of age (60%–70%). Its clinical presentation is usually acute or subacute, with cough, chest pain and fever (70% of patients), malaise, fatigue, anorexia, weight loss, sweating and varying degrees of dyspnea. PE is usually unilateral (95%), of small to moderate size, although it can be massive on occasions (12%–18%) and sometimes loculated (30%). Coexisting lung disease can be determined on chest X-ray (4%–20%) or CT (40%–85%).
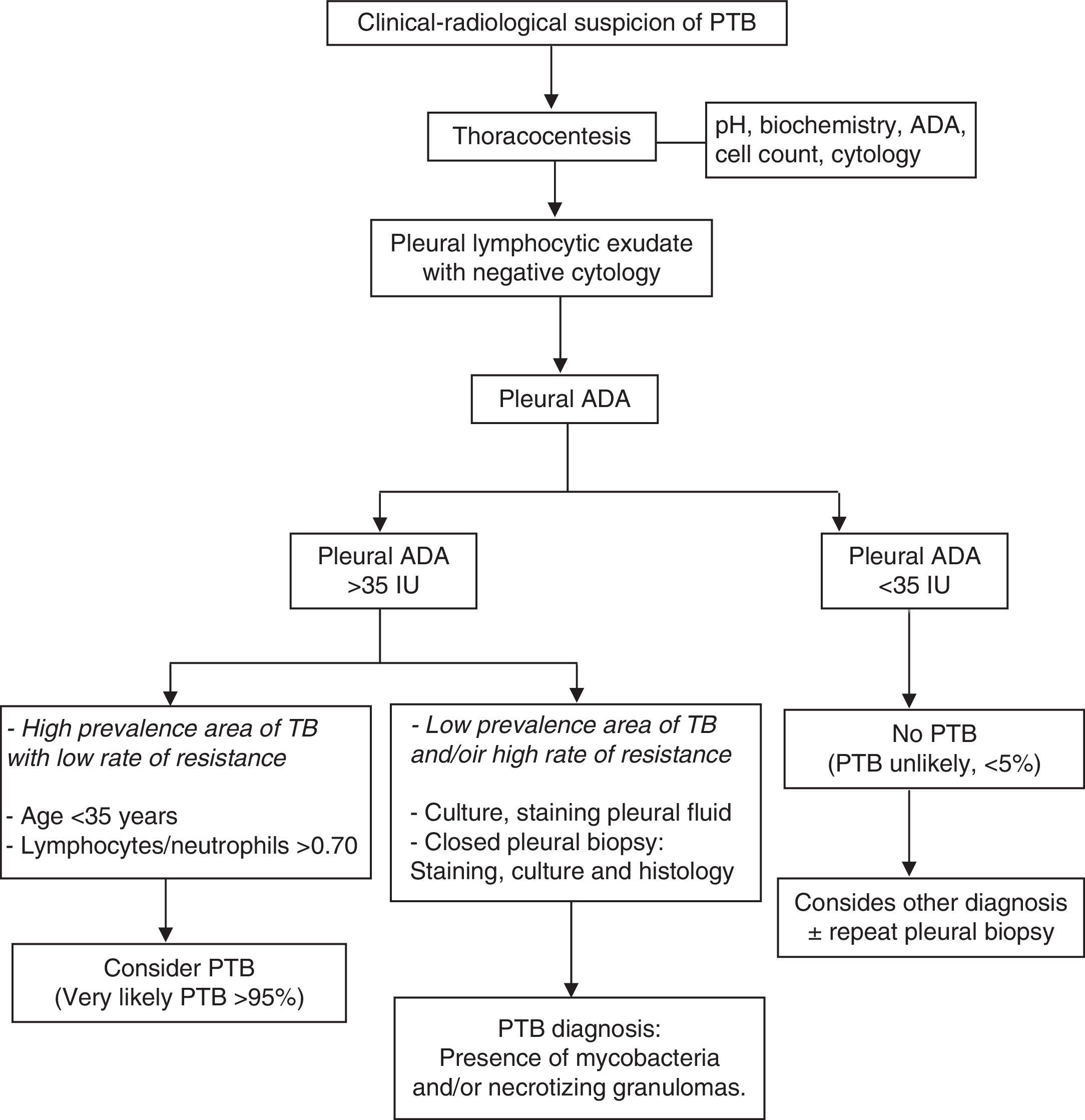
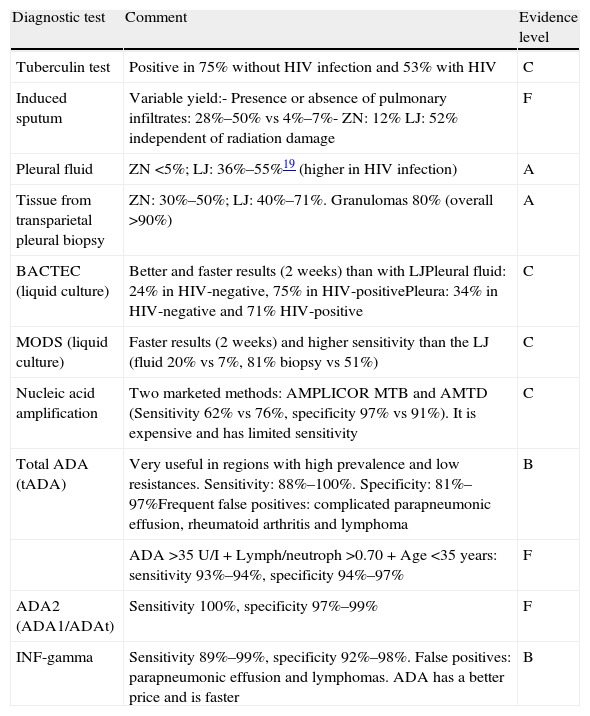
DiagnosisThe characteristics of the methods for PTB diagnosis are shown in Table 2. PF analysis shows a predominantly lymphocytic pleural exudate in most cases (90%), occasionally polymorphonuclear in the first days, with high levels of adenosine deaminase (ADA), without eosinophilia and less than 5% of mesothelial cells (A).20 PTB diagnosis is confirmed with the isolation of Mycobacterium tuberculosis in PF or pleural tissue. BACTEC provides a greater yield and faster results (2 weeks), compared to Löwenstein–Jensen (LJ) culture.21 The microscopic observation drug susceptibility (MODS) assay provides faster results (2 weeks) and higher sensitivity than LJ (20% in PF versus 7%; 81% in pleural tissue samples compared to 51%).21 (C) Nucleic acid amplification tests have low sensitivity for the diagnosis of PTB (this is possibly related to inhibitors in pleural fluid or intracellular sequestration of mycobacteria) and high specificity.22
Diagnostic Methods in Pleural Tuberculosis.
| Diagnostic test | Comment | Evidence level |
| Tuberculin test | Positive in 75% without HIV infection and 53% with HIV | C |
| Induced sputum | Variable yield:- Presence or absence of pulmonary infiltrates: 28%–50% vs 4%–7%- ZN: 12% LJ: 52% independent of radiation damage | F |
| Pleural fluid | ZN <5%; LJ: 36%–55%19 (higher in HIV infection) | A |
| Tissue from transparietal pleural biopsy | ZN: 30%–50%; LJ: 40%–71%. Granulomas 80% (overall >90%) | A |
| BACTEC (liquid culture) | Better and faster results (2 weeks) than with LJPleural fluid: 24% in HIV-negative, 75% in HIV-positivePleura: 34% in HIV-negative and 71% HIV-positive | C |
| MODS (liquid culture) | Faster results (2 weeks) and higher sensitivity than the LJ (fluid 20% vs 7%, 81% biopsy vs 51%) | C |
| Nucleic acid amplification | Two marketed methods: AMPLICOR MTB and AMTD (Sensitivity 62% vs 76%, specificity 97% vs 91%). It is expensive and has limited sensitivity | C |
| Total ADA (tADA) | Very useful in regions with high prevalence and low resistances. Sensitivity: 88%–100%. Specificity: 81%–97%Frequent false positives: complicated parapneumonic effusion, rheumatoid arthritis and lymphoma | B |
| ADA>35U/I+Lymph/neutroph>0.70+Age<35 years: sensitivity 93%–94%, specificity 94%–97% | F | |
| ADA2 (ADA1/ADAt) | Sensitivity 100%, specificity 97%–99% | F |
| INF-gamma | Sensitivity 89%–99%, specificity 92%–98%. False positives: parapneumonic effusion and lymphomas. ADA has a better price and is faster | B |
ZN, Ziehl–Neelsen; LJ: Löwenstein–Jensen; MODS, microscopic observation of drug susceptibility; ADA, Adenosin deaminase; ADA2, ADA isoenzyme; ADA1, ADA isoenzyme; INF-gamma, interferon gamma.
Analysis of pleural tissue obtained by transmural pleural biopsy may show granulomas, providing a presumptive diagnosis. Its sensitivity is higher than 80% and presence of acid-fast bacilli (30%–50%) or M. tuberculosis (40%–71%) in culture increases its yield (90%) and confirms the diagnosis, (A).20 Diagnostic thoracoscopy is indicated only in case of persistent clinical suspicion and negative results in the clinical studies performed (F).
ADA levels are elevated in PF, with a sensitivity of 88–100% and specificity of 81%–97%,23 and the most accepted cutoff level is 35IU (B). Yields are similar in human immunodeficiency virus (HIV) carriers, even those with very low CD4 counts.24 Yield improves in patients under 35 years with a lymphocyte/neutrophil ratio in PF>0.70 (sensitivity 93%–94%, specificity 94%–97%). ADA false positives occur mainly in complicated PPPE (especially empyema) and lymphoma, and include other less frequent entities such as rheumatoid arthritis and some carcinomas. In PTB, increases in ADA are the result of mainly monocyte-derived macrophages ADA2 isoenzyme. Hence, yields superior to those of ADA are described for ADA1/total ADA ratio values<0.42 (total ADA=ADA1+ADA2), with sensitivity 100% and specificity 97%–99%.25 (F)
Levels of IFNγ derived from T-lymphocyte (CD4) activation are also increased in PTB and the diagnostic yield is as high as that of ADA20 (sensitivity 89%–99%, specificity 92%–98%), with a variable cutoff level depending on the method and units (B). False positives have been described in empyema and lymphomas. ADA has been recommended on the basis of its lower price and the greater speed.
The combination of diagnostic tests for lymphocytic PE, including patient age, ADA levels, PF cultures and staining, mycobacteria cultures and histological analysis of pleural transmural biopsy, along with the therapeutic response, constitutes an appropriate diagnostic approach (Fig. 4).
Treatment of PTB does not differ from that described for pulmonary tuberculosis: 2 months with rifampicin+isoniazid+ethambutol+pyrazinamide, and the next 4 months with isoniazid+rifampicin (A). Concomitant pleural drainage may reduce the symptomatic period, but no evidence of any improvement in mid to long term outcomes is available.26
Intrapleural fibrinolytics in loculated PTB may facilitate PE resolution and reduce residual pleural thickening (>10mm). However, pleural thickening is observed in less than 20% of patients after one year of follow-up and the functional impact is low in most cases (G). Corticosteroids, initiated two weeks after TB treatment, may be clinically useful in severe cases for shortening the symptomatic period, but have not shown long-term usefulness (G).
Tuberculous empyema is rare, but often requires pleural decortication due to functional disability and treatment failure caused by a trapped and often loculated lung, with thickened and even calcified pleura, which may hinder drug penetration (H).
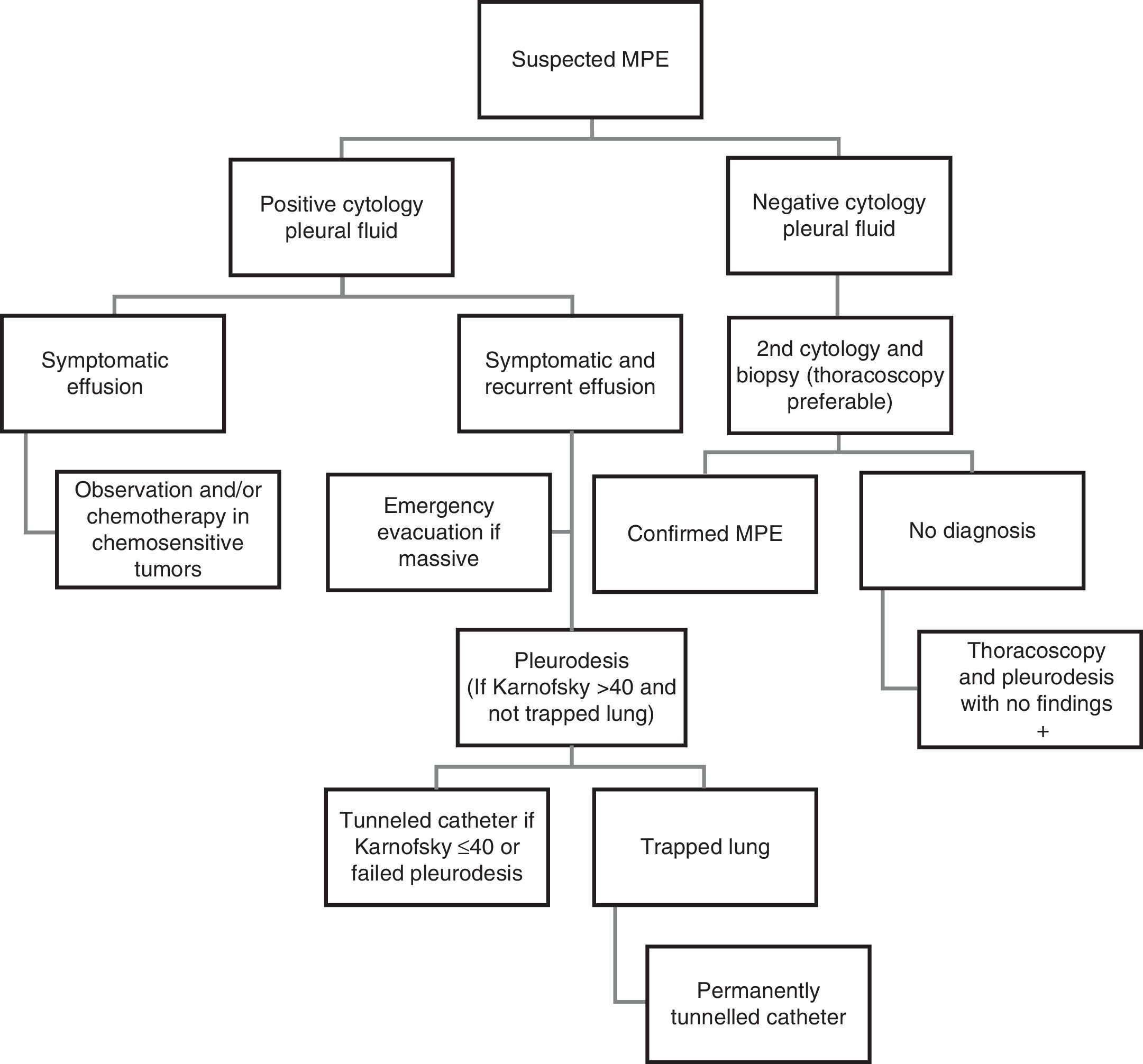
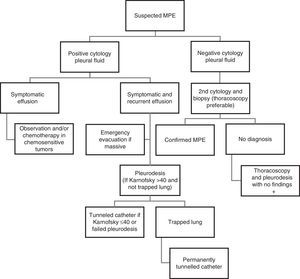
Malignant Pleural EffusionMalignant pleural effusion (MPE) represents between 15% and 35% of all PE and is one of the leading causes of pleural exudate, but neoplastic involvement of the pleura without PE is also possible and should be considered. Median survival is usually between 4 and 6 months. Most MPE are produced by pleural metastases, especially of lung (more than a third of the cases), and breast tumors. If the patient has been exposed to asbestos, mesothelioma can be the third leading cause of MPE, but lymphoma and ovarian tumors, among others, should also be considered. The most probable cause of PE in a woman with known breast cancer (ipsilateral or contralateral to the affected breast) is metastasis.
The main mechanism of MPE is increased vascular permeability, often associated with obstruction of lymphatic drainage at a pleural and/or mediastinal level. “Paraneoplastic PE” may also occur due to causes in which there is no direct pleural infiltration by the tumor, such as obstructive pneumonitis or atelectasis, pulmonary embolism, mediastinal lymph blockade, obstruction of the thoracic duct (chylothorax), superior vena cava syndrome, tumor involvement of the pericardium, radiochemotherapy-induced toxicity, or hypoalbuminemia. The recommendations for intervention in patients with suspected MPE are shown in Fig. 5.
Clinical and Radiological ManifestationsMost MPE begin with exertional dyspnea that progresses as the effusion enlarges and compresses the lung and the diaphragm. If persistent, severe pain is present, mesothelioma should be suspected, especially in subjects with a history of asbestos exposure.
Radiologically, the following should be taken into account:
- -
MPE often occupies more than half of the hemithorax.
- -
If bilateral (more common in breast and ovary metastases), the apparent size of the heart is not increased (except in the case of very advanced stages of pericardial effusion).
- -
If the mediastinum is centered in presence of a massive unilateral PE, proximal bronchial obstruction, mediastinal fixation due to tumor and/or lymphadenopathy, or extensive pleural infiltration (mesothelioma and/or “trapped lung”) should be suspected.
- -
CT with contrast is recommended, on which PE may be observed with no other finding in more than 50% of cases. Malignancy should be suspected if pleural nodules, diffuse thickening of the parietal pleura greater than 1cm, “circumferential” thickening of the entire pleura (including the mediastinum; this appears frequently in advanced mesothelioma), or liver metastases associated with the PE are observed.
- -
Thoracic ultrasound may exceed 95% specificity for MPE if marked thickening of parietal or visceral pleura, or nodules on the diaphragm surface are observed.27
Hemorrhagic appearance reinforces suspicion of malignancy. The fluid is an exudate in over 95% of cases. More than 80% are predominantly lymphocytic, with ADA levels below 35U/L in about 95% of cases. Glucose levels are less than 60mg/dL, and in about 30% of cases, pH is 7.30, indicating advanced disease and an increased likelihood of positive biopsy and cytology. Note that the determination of tumor markers in PF is not recommended as a routine method (C). The yield of cytology is approximately 60%; higher in breast and ovarian cancers, compared to lymphomas, sarcomas and mesotheliomas,28 and when 60ml of PF or more is sent to the laboratory. Repeating cytology more than twice is not profitable (B), and biopsy is recommended when a second cytology is needed.29 PF examination by flow cytometry can be helpful if lymphoma is suspected. Cell block techniques or immunocytochemical staining are clinically useful. Unless instructed otherwise, PF samples for cytology can be collected in citrated tubes to prevent clotting, but other media should not be used, because of the risk of interfering with laboratory techniques.
Pleural Needle BiopsyAt approximately 50%, the sensitivity of pleural needle biopsy in MPE is lower than PF cytology. However, sensitivity increases in the presence of glucose and low pH in the PF. Combined use of cytology and biopsy improves diagnostic yield. This is especially recommended in case of doubt between tuberculosis and malignancy, as it is highly useful in tuberculous pleuritis and, unlike thoracoscopy, can be performed on an outpatient basis. If marked diffuse pleural thickening with sparse liquid or large nodules in the parietal pleura are observed on ultrasound or CT, CT-guided needle biopsy can achieve a yield of 85% (B).30
ThoracoscopyThis is the procedure of choice when the effusion persists for more than two weeks and cytology is negative. Its diagnostic yield is over 95% (B). Thoracoscopy allows visual control of large biopsies of the parietal and visceral pleura (for immunohistochemistry and other studies). It also facilitates assessment of the tumor burden in the pleural cavity and pleurodesis can be performed during the same procedure. Thoracoscopy may be performed in a properly equipped respiratory endoscopy unit, with local anesthesia and analgesia/sedation without intubation.
TreatmentSeveral options are available for the management of MPE.
Systemic TherapyChemotherapy can be effective in controlling MPE when associated with lymphoma, small-cell lung carcinoma, or breast cancer. However, early pleurodesis is recommended in case of rapid relapse, in order to prevent deterioration of the patient or the development of a trapped lung, which would prevent lung reexpansion and the symphysis between visceral and parietal pleura (B).
Therapeutic ThoracentesisThis should be urgently performed in patients with massive PE and contralateral mediastinal shift, but evacuation should be slow to avoid reexpansion pulmonary edema. Particular care should be taken if the mediastinum is centered in presence of massive PE. In these cases it is advisable to monitor pleural pressure during fluid evacuation.31 Therapeutic thoracentesis as a single therapeutic measure to control MPE is not recommended, except in patients with short life expectancy (less than 1 month). The use of a thin catheter (10–14F) is preferred, with pleurodesis if the lung is not trapped (A).32
PleurodesisIntrapleural application of an irritant substance causes intense inflammation that leads to fibrosis, sclerosis between parietal and visceral pleura and obliteration of the pleural space. Pleurodesis is indicated when PE is symptomatic (dyspnea) with a tendency to recur after therapeutic thoracentesis, Karnofsky index is greater than 40, and the lung is potentially reexpandable after therapeutic thoracentesis, having ruled out proximal bronchial obstruction and/or trapped lung.33
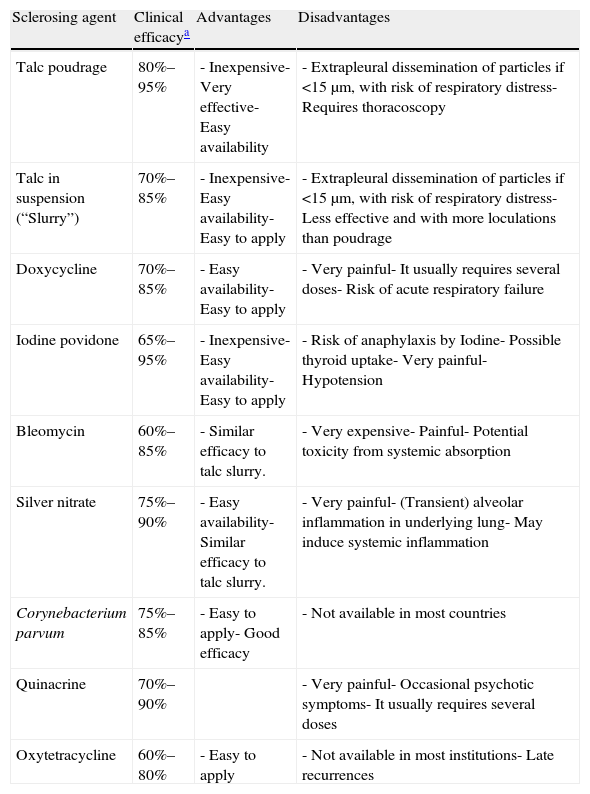
Talc is the agent of choice for pleurodesis (A) and, in order to minimize the risk of complications, it should be free of contaminants (asbestos, etc.) and the size of its particles should be greater than 15μm (B).34 When thoracoscopy is available, it allows combined diagnosis and treatment (pleurodesis with talc powder, poudrage). Talc can also be used in suspension (in saline solution) through a drainage tube (slurry), and this option is preferable for patients with contralateral pleuro-pulmonary disease, who would be unable to tolerate the single lung collapse necessary for thoracoscopy, or those with low Karnofsky index. Doxycycline can also instilled in these cases, always taking into account the need for careful analgesia. Instillation of bleomycin, povidone iodine and silver nitrate have been proposed as alternatives for pleurodesis, but none of them is without complications, and their effectiveness is slightly lower than that of talc. Table 3 shows the characteristics of different sclerosing agents along with their clinical effectiveness.35
Agents Most Frequently Used for Pleurodesis in Clinical Practice.
| Sclerosing agent | Clinical efficacya | Advantages | Disadvantages |
| Talc poudrage | 80%–95% | - Inexpensive- Very effective- Easy availability | - Extrapleural dissemination of particles if <15μm, with risk of respiratory distress- Requires thoracoscopy |
| Talc in suspension (“Slurry”) | 70%–85% | - Inexpensive- Easy availability- Easy to apply | - Extrapleural dissemination of particles if <15μm, with risk of respiratory distress- Less effective and with more loculations than poudrage |
| Doxycycline | 70%–85% | - Easy availability- Easy to apply | - Very painful- It usually requires several doses- Risk of acute respiratory failure |
| Iodine povidone | 65%–95% | - Inexpensive- Easy availability- Easy to apply | - Risk of anaphylaxis by Iodine- Possible thyroid uptake- Very painful- Hypotension |
| Bleomycin | 60%–85% | - Similar efficacy to talc slurry. | - Very expensive- Painful- Potential toxicity from systemic absorption |
| Silver nitrate | 75%–90% | - Easy availability- Similar efficacy to talc slurry. | - Very painful- (Transient) alveolar inflammation in underlying lung- May induce systemic inflammation |
| Corynebacterium parvum | 75%–85% | - Easy to apply- Good efficacy | - Not available in most countries |
| Quinacrine | 70%–90% | - Very painful- Occasional psychotic symptoms- It usually requires several doses | |
| Oxytetracycline | 60%–80% | - Easy to apply | - Not available in most institutions- Late recurrences |
Clinical efficacy: the sum of complete and partial successes in which, although small recurrence of effusion may be observed, therapeutic thoracentesis is not required during the whole progression, once the sclerosing agent is applied.35
In recent years, the use of these catheters has become popular to control malignant pleural effusions, and are even used as an alternative to chemical pleurodesis.36–41 They are particularly indicated in patients with trapped lung, those with short life expectancy or those in whom a previous attempt to pleurodesis has failed (B).42 Although pleural symphysis occurs spontaneously in approximately half of the patients with this type of catheter, the possibility of instilling talc by this route to complement the therapeutic effect has been raised. The main problems lie in the costs, the risk of infection, neoplastic invasion in the insertion point and chronic protein loss from repeated PF evacuations.
PleurectomyThis may be proposed in very exceptional cases, especially in patients with mesothelioma and in those in good general condition with pleurodesis failure. Pleurectomy is performed with video-assisted thoracoscopic surgery. It is a very invasive procedure involving significant morbidity.
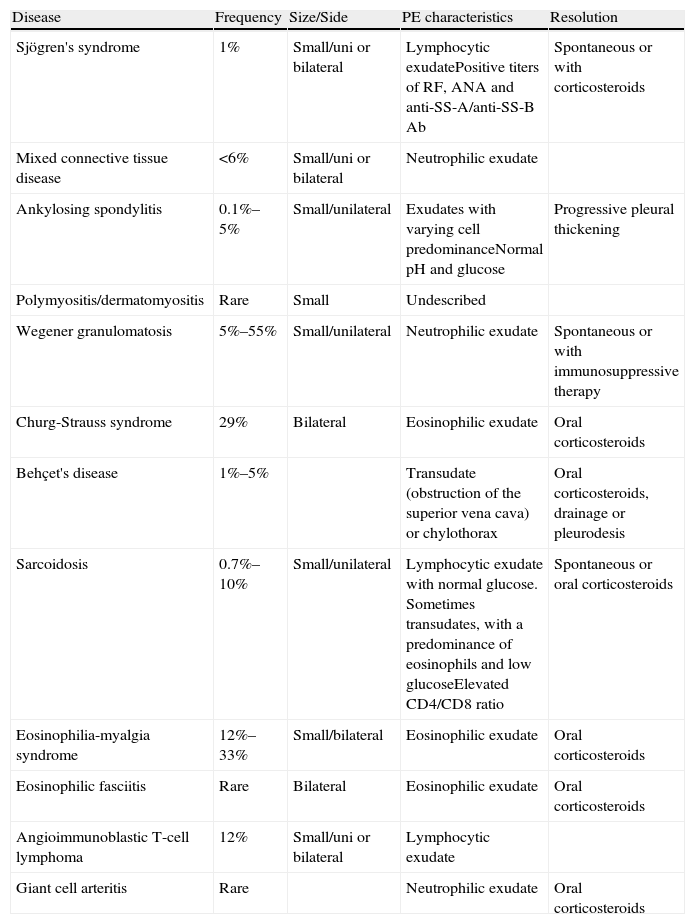
Pleural Effusion in Systemic DiseasesThe incidence of PE in systemic diseases is approximately 1%. The most important characteristic, especially in connective tissue diseases, is the increase in capillary permeability, as a result of direct infiltration of the pleura or an immune mechanism. PE may also occur as a result of the renal or cardiac involvement that may accompany these entities, or from thromboembolic disease or drug use. The two most frequent conditions include rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). Other diseases are summarized in Table 4.43
Characteristics of Pleural Effusion in Rarer Systemic Diseases.
| Disease | Frequency | Size/Side | PE characteristics | Resolution |
| Sjögren's syndrome | 1% | Small/uni or bilateral | Lymphocytic exudatePositive titers of RF, ANA and anti-SS-A/anti-SS-B Ab | Spontaneous or with corticosteroids |
| Mixed connective tissue disease | <6% | Small/uni or bilateral | Neutrophilic exudate | |
| Ankylosing spondylitis | 0.1%–5% | Small/unilateral | Exudates with varying cell predominanceNormal pH and glucose | Progressive pleural thickening |
| Polymyositis/dermatomyositis | Rare | Small | Undescribed | |
| Wegener granulomatosis | 5%–55% | Small/unilateral | Neutrophilic exudate | Spontaneous or with immunosuppressive therapy |
| Churg-Strauss syndrome | 29% | Bilateral | Eosinophilic exudate | Oral corticosteroids |
| Behçet's disease | 1%–5% | Transudate (obstruction of the superior vena cava) or chylothorax | Oral corticosteroids, drainage or pleurodesis | |
| Sarcoidosis | 0.7%–10% | Small/unilateral | Lymphocytic exudate with normal glucose. Sometimes transudates, with a predominance of eosinophils and low glucoseElevated CD4/CD8 ratio | Spontaneous or oral corticosteroids |
| Eosinophilia-myalgia syndrome | 12%–33% | Small/bilateral | Eosinophilic exudate | Oral corticosteroids |
| Eosinophilic fasciitis | Rare | Bilateral | Eosinophilic exudate | Oral corticosteroids |
| Angioimmunoblastic T-cell lymphoma | 12% | Small/uni or bilateral | Lymphocytic exudate | |
| Giant cell arteritis | Rare | Neutrophilic exudate | Oral corticosteroids |
PE, pleural effusion; FR, rheumatoid factor, ANA, antinuclear antibodies; Ab: antibodies.
Pleural involvement is the most common intrathoracic manifestation of rheumatoid arthritis (RA) and occurs in 5% of patients. Although RA is more common in women, the majority of rheumatoid PEs occur in middle-aged males (80%), with high titers of rheumatoid factor, rheumatoid nodules, and presence of both HLA-B8 and Dw3. PE in these cases is usually small, unilateral (70%), on the left side, and asymptomatic. It usually occurs years after RA diagnosis and may be transient, recurrent or chronic. The appearance of the fluid can be serous, milky, hemorrhagic and even purulent. The biochemical characteristics of chronic PE (80% of cases) are usually pH <7.20, with a low glucose content (80% below 50mg/dl), pleura/serum glucose ratio <0.5, elevated LDH (>1000U/L), rheumatoid factor titer higher than 1/320 (generally higher in the fluid than in blood), and low levels of total hemolytic complement and complement components. However, pH and glucose levels are usually normal in acute PE. In countries with a low incidence of tuberculosis, chronic rheumatoid effusions are the most common cause of pseudochylothorax, but chylothorax and empyema have also been described. Pleural biopsy is usually not diagnostic. Usually, rheumatoid PEs do not require treatment, but sometimes repeated therapeutic thoracentesis are needed to prevent pleural thickening and trapped lung.44
Systemic Lupus ErythematosusPleural involvement may be the disease presentation in 5% of cases, while 30%–50% will develop symptomatic pleural inflammation throughout their illness. PEs tend to be bilateral (50%), small and rarely associated with underlying lung disease. They behave like typical exudates and there are no definitive tests to differentiate them from other effusion types. Low complement levels and high titers of antinuclear antibodies (ANA) (>1/160) in PF are suggestive but not conclusive, because some MPE, especially lymphomas, may also present high titers. A recent study shows that the determination of ANA in PF does not provide additional information compared to ANA measurement in serum. Therefore, PF determination is recommended only in patients with SLE and a PE of uncertain etiology, since the absence of ANAs in the fluid would act against the diagnosis of lupus pleuritis.45 Presence of lupus erythematosus cells is highly specific, although this test is rarely performed due its long preparation time. In pleural biopsy a specific immunofluorescence pattern can be observed, characterized by nuclear staining of pleural cells either with anti-IgM, anti-IgG or anti-C3. In drug-induced lupus, PE can also be present, and usually disappears once the medication is withdrawn. In most cases, effusions by SLE or drug-induced lupus respond well to nonsteroidal antiinflammatory drugs (NSAIDs) or low-doses of oral corticosteroids.46
Pleural Effusion Due Cardiovascular DiseasePleural Effusion Due to Heart FailureIt is probably the most common cause of PE. On standard X-ray, approximately 60% of these PEs are bilateral, 30% unilateral in the right side and 10% unilateral in the left side.47 The PE occupies only a third (sometimes less) of the hemithorax in more than 80% of cases.47 PF is accumulated in the fissures and may simulate a lung tumor. PF analysis is not necessary in a typical clinical–radiological context. Thoracentesis is only indicated in: (1) unilateral PE, particularly if there is no cardiomegaly; (2) patients with fever or pleuritic pain; and (3) persistent PE despite diuretic therapy.
In most patients with heart failure, PE is solved with diuretics. In the few cases in which cardiac PE is refractory to conventional medical treatment, repeated therapeutic thoracentesis, pleurodesis or intrapleural insertion of a permanent catheter may be used. Unilateral pleurodesis has the risk of potentially increasing PE on the opposite hemithorax. Although experience is limited, permanent intrapleural catheters control dyspnea in all patients, and it can be removed after a couple of months in half of the cases, mainly thanks to the development of a spontaneous pleurodesis.48
Effusion After Coronary Revascularization SurgeryIn the immediate postoperative period after revascularization by coronary artery bypass surgery, most patients have small PEs that gradually resolve.49 However, the incidence of PEs occupying more than a quarter of the hemithorax is 10%. These PEs of moderate or large size produce dyspnea and are usually located on the left side; if bilateral, they predominate on the left side. Thoracentesis should always be performed, because the differential diagnosis includes heart failure, pulmonary embolism, chylothorax or infectious PE. Symptomatic PEs within the first 30 days after surgery are usually due to surgical trauma and the PF is a frequently bloody and eosinophilic exudate. PEs that reach their maximum size more than one month after surgery are lymphocytic exudates whose pathogenesis is probably of immune origin that may be a limited variation of post-cardiac injury syndrome. Treatment includes therapeutic thoracentesis that needs to be repeated twice or more in 20% of cases.
Pericardial DiseasesPost-cardiac injury syndrome is the occurrence of pericarditis (with or without pericardial effusion), days, weeks or months after an acute myocardial infarction (Dressler's syndrome), cardiac surgery with pericardiotomy, pericardial injury or even a minor precipitating factor such as percutaneous coronary intervention, the insertion of a pacemaker or radiofrequency ablation. The incidence of Dressler's syndrome is less than 1% and that of post-pericardiotomy syndrome is 15%. The mechanism is immune-mediated and patients typically present with pleuritic and/or pericardial pain, fever and elevated acute phase reactants, along with small unilateral pleural exudates predominantly in the left side and, occasionally, pulmonary infiltrates. Treatment consists of aspirin (Dressler's syndrome), NSAIDs (other cases) and, in the case of lack of response or relapse, corticosteroids may be administered. Prophylactic use of colchicine in the immediate postoperative period (0.5mg/12h for 1 month) significantly reduces the risk of pleuro-pericardial effusion after cardiac surgery.50
PE is associated with at least a quarter and over a half of acute and constrictive pericarditis cases, respectively. In the first case, PEs are mostly small (≤1/3 of the hemithorax) and unilaterally left, while in the second case, bilateral PEs are predominant. Biochemical analysis reveals exudative nature almost always. Acute pericarditis is treated with NSAIDs, which must be combined with colchicine for 3 months (0.6mg/day if body weight<70kg or 1mg/day if body weight>70kg) to reduce the risk of persistent or recurrent disease.
Pulmonary ThromboembolismChest X-ray reveals PE in a third of patients with pulmonary embolism, although more sensitive techniques, such as ultrasound or CT, reveal PE in half of cases.51 PE occupies less than a third of the hemithorax in 90% of patients, and is unilateral in 85% of cases. In 20% of subjects, particularly if the diagnosis is delayed more than 10 days after the onset of symptoms, PE is loculated.51 Due to the small size of the PEs and the immediate anticoagulant therapy initiated when this entity is clinically suspected, only one third of patients undergo diagnostic thoracentesis. PF is bloody in half of cases, with biochemical characteristics of an exudate, and varying prevalence of neutrophils or lymphocytes. The treatment of embolism is not modified by the presence of PE.
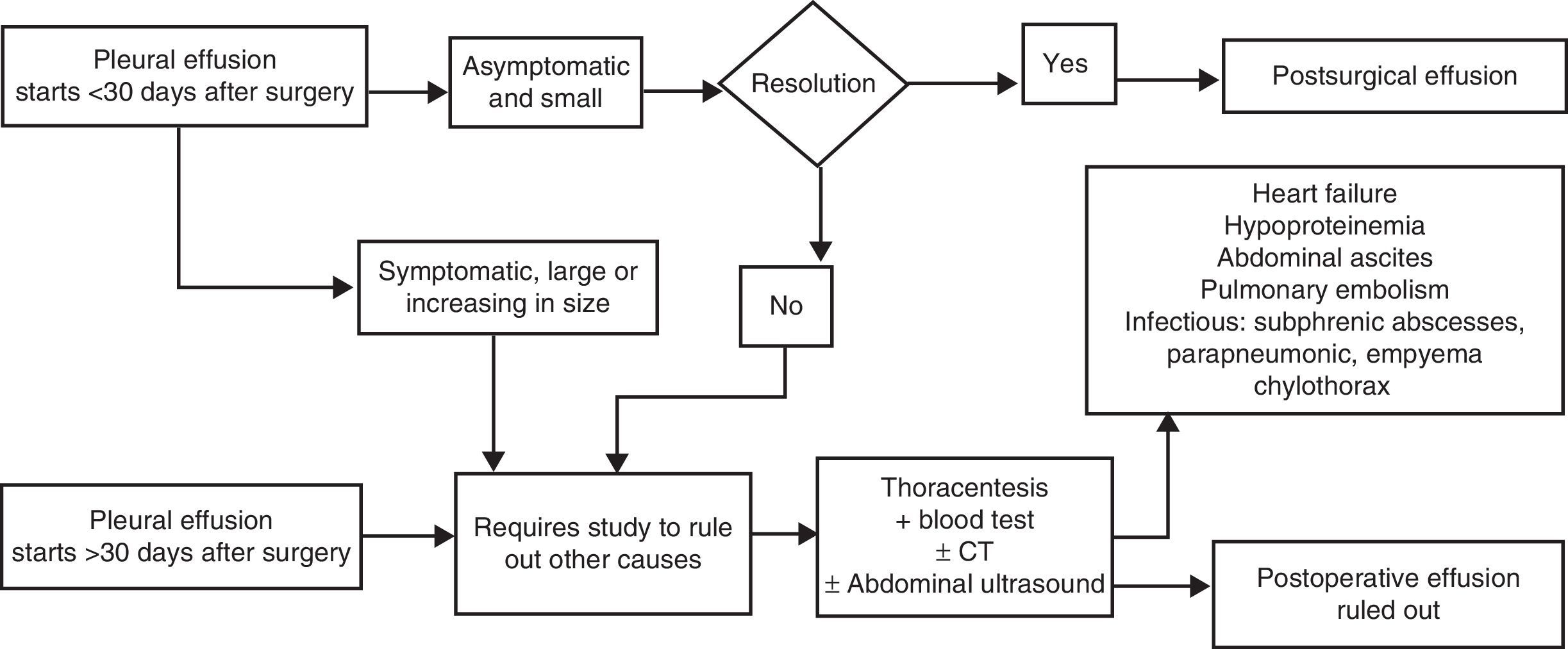
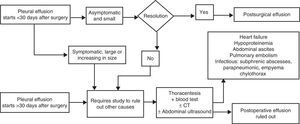
Postoperative Pleural EffusionEmergence of PE after abdominal or thoracic surgery (either lung resection, lung transplantation or heart surgery), without any other reason justifying suspicion of embolism, infection, heart failure, chylothorax or trapped lung, is frequent in the immediate postoperative period (day 1–4 after surgery). Its incidence varies between 40% and 80%, depending on the diagnosis being made by chest X-ray or ultrasound. These immediate postsurgical PE may be unilateral or bilateral; they are usually small and asymptomatic, and resolve spontaneously within the first month (thoracentesis is not necessary). However, they occasionally persist for more than 3 months, but usually remaining smaller than a quarter of hemithorax.
In cardiac or thoracic surgery, postoperative PE is usually a neutrophilic exudate during the first month, and subsequently lymphocytic. It is caused by manipulation of the pleural cavity and/or post-cardiac damage (in this case associated with fever, dyspnea and leukocytosis).49 PE after abdominal surgery is most often of a transudative nature, favored by previous ascites, hypoalbuminemia, etc., although sometimes diaphragmatic irritation also results in exudate.52
PE associated with symptoms, such as fever or pain, after abdominal or thoracic surgery, late appearance of PE, persistence for more than 30 days, or increased size over time, require the exclusion of other typical complications during postoperative period, such as pleural bleeding, embolism, infection, chylothorax, heart failure, trapped lung, abscess or subdiaphragmatic hematoma, among others (Fig. 6). Postsurgical PE does not require specific treatment, and the need for drainage depends on the respiratory compromise it produces in the patient.
In lung transplant patients with occurrence of PE at any point in their clinical course or increased postoperative PE, even if asymptomatic, requires ruling out graft rejection by transbronchial lung biopsy. Thoracentesis is indicated only once rejection has been ruled out, and other causes such as infection or neoplasia will be explored.53
Pleural Effusion Due to Drug UsePE secondary to drug use, usually associated with parenchymal involvement, is a rare entity. The list of potentially causative agents is extensive (www.pneumotox.com), so careful patient history is imperative. Those secondary to the use of amiodarone, nitrofurantoin, methysergide and bromocriptine are among the most common. There is no specific data for diagnosis, which should always be performed after excluding other possible etiologies. PE may be eosinophilic and sometimes accompanied by peripheral eosinophilia. Pleural biopsy typically shows unspecific inflammation.20,54 Temporal relationship between drug intake and the presence of PE should be established for diagnosis. PE disappearance after drug interruption would confirm the causal relationship and therefore the diagnosis (C).20,54,55
Pleural Effusion in Benign Gynecological DiseasesMeigs’ syndrome, endometriosis or ovarian hyperstimulation syndrome are rare causes of PE, usually accompanied by ascitis.20,55Meigs’ syndrome is characterized by the simultaneous presence of ascites and pleural effusion in association with a benign ovarian solid tumor, although sometimes association with tumors of low grade malignancy, such as granular cell tumors, may occur. Diagnosis is confirmed when, after removal of the primary tumor, ascites and PE are resolved, but resolution may take several weeks. Advanced stage endometriosis should be included in the differential algorithm of PE in women of childbearing age, as this can be a very rare cause of hemothorax.20,55 Note that, in these two entities, elevations of tumor markers such as CA-125 may be observed, and diagnosis of disseminated malignancy should not be assumed.20Ovarian hyperstimulation syndrome refers to the clinical syndrome of ovarian enlargement due to the existence of multiple cysts and fluid accumulation in the extravascular space. History of hormonal treatment for recent fertilization and exclusion of other causes of PE are required for diagnosis. Treatment of this entity is based on electrolyte control, and prevention of complications such as thromboembolic disease. If PE produces dyspnea, therapeutic thoracentesis is indicated (D).20,55
Pleural Effusion in Benign Digestive DiseasePancreatic and liver disease, intra-abdominal abscesses, esophageal perforation or abdominal surgical procedures can produce PE with some frequency, although its detection depends on the imaging technique used (X-ray, ultrasound or chest CT)20,55 The clinical picture is usually characterized by the digestive process causing it and treatment will depend on its control. Diagnostic thoracentesis is usually indicated if complicated PE or other possible etiologies are suspected (D). Although elevated amylase in the PF is very characteristic of PE secondary to pancreatic pathology or esophageal perforation, this can also occur in malignant PE and, less frequently, in tuberculous PE.20,56 If PE is very symptomatic or not resolved after control of gastrointestinal disease, therapeutic thoracentesis or pleural drainage may be indicated (D).
Hepatic hydrotorax, a complication in about 6% of cirrhosis cases with ascites, is initially treated with salt restriction and combined furosemide and spironolactone. One or more therapeutic thoracenteses may be needed if the PE is large or massive. Nearly a third of hepatic hydrothoraces become refractory to diuretic treatment.57 Liver transplant should be indicated for these patients, as the only measure associated with longer survival. While the patient is on the waiting list or if circumstances contraindicating transplantation are present, refractory hepatic hydrothorax may be managed with a percutaneous intrahepatic portosystemic shunt or a permanent intrapleural catheter.57 However, pleurodesis is not considered a good option as it fails in most cases.
The characteristics of PEs produced by the most commonly associated gynecological and digestive diseases are summarized in Table 5.
Most Common Features of Pleural Effusion Associated With Benign Gynecological and Digestive Diseases.
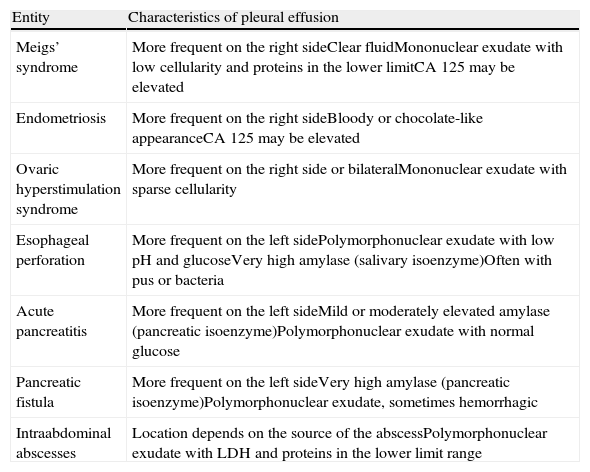
| Entity | Characteristics of pleural effusion |
| Meigs’ syndrome | More frequent on the right sideClear fluidMononuclear exudate with low cellularity and proteins in the lower limitCA 125 may be elevated |
| Endometriosis | More frequent on the right sideBloody or chocolate-like appearanceCA 125 may be elevated |
| Ovaric hyperstimulation syndrome | More frequent on the right side or bilateralMononuclear exudate with sparse cellularity |
| Esophageal perforation | More frequent on the left sidePolymorphonuclear exudate with low pH and glucoseVery high amylase (salivary isoenzyme)Often with pus or bacteria |
| Acute pancreatitis | More frequent on the left sideMild or moderately elevated amylase (pancreatic isoenzyme)Polymorphonuclear exudate with normal glucose |
| Pancreatic fistula | More frequent on the left sideVery high amylase (pancreatic isoenzyme)Polymorphonuclear exudate, sometimes hemorrhagic |
| Intraabdominal abscesses | Location depends on the source of the abscessPolymorphonuclear exudate with LDH and proteins in the lower limit range |
Asbestos exposure may cause multiple manifestations of pleural pathology, several of which often coexist in the same patient. These include pleural plaques, diffuse pleural fibrosis, and benign asbestos-related PE. The latter is characterized by a moderate or small unilateral exudate, with serous and sanguineous characteristics, and often with eosinophilia. Diagnosis is based on suspicion and the exclusion of other pathologies, for which follow-up of at least 3 years is required. It tends to resolve spontaneously, with relapse in a third of cases.
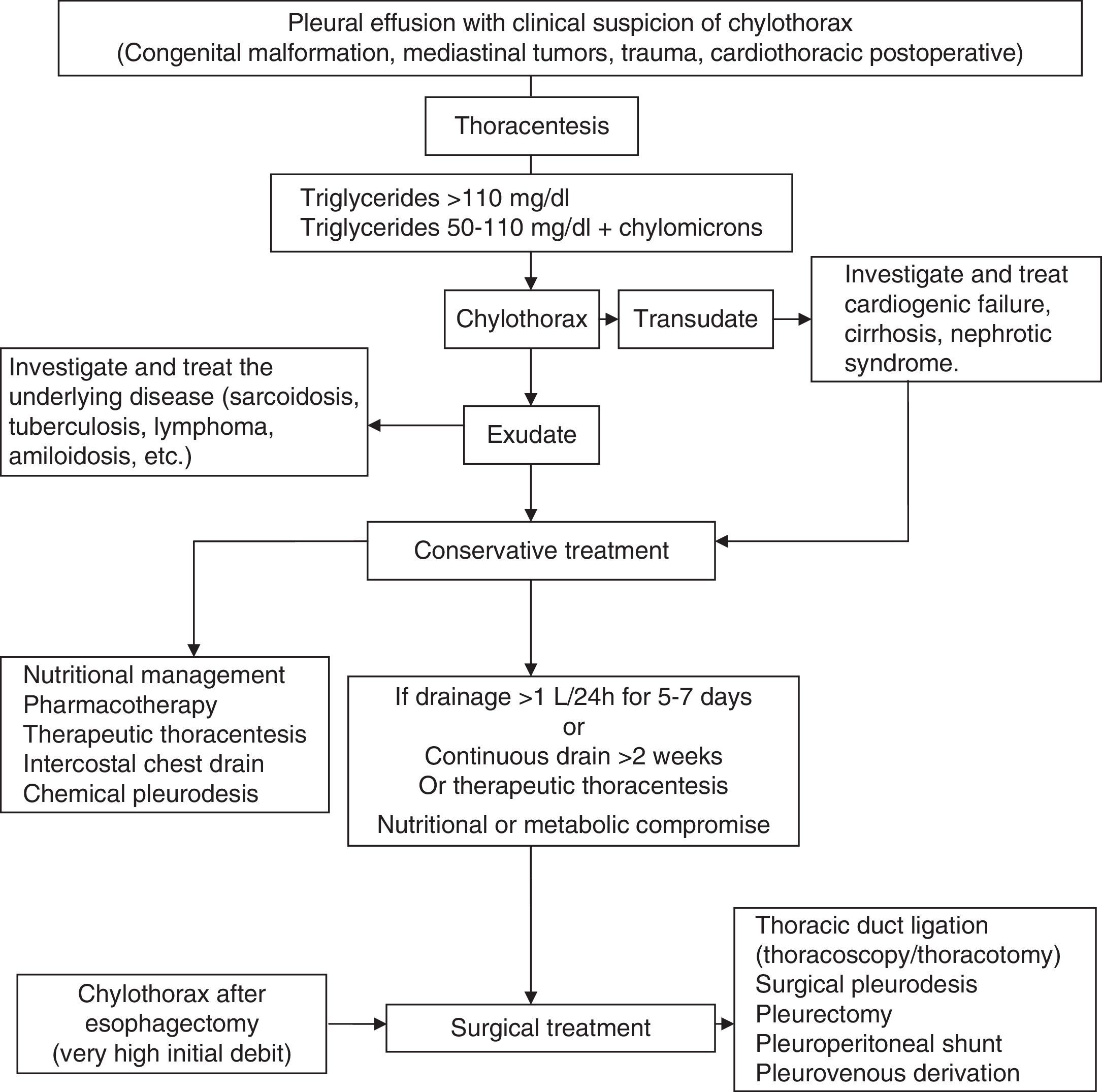
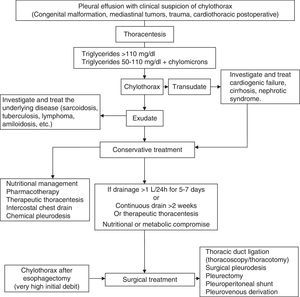
ChylothoraxIt arises from the accumulation of lymph fluid from intestinal origin (chyle) in the chest cavity as a result of the rupture or obstruction of the thoracic duct, or the passage of accumulated chyle into the peritoneal space (chylous ascites). The most common causes are malignancy and trauma.1 Diagnosis is established by triglyceride levels in PF greater than 110mg/dl. Triglyceride levels below 50mg/dl exclude a CHT with reasonable certainty in the absence of a causative diet or parenteral nutrition. If the level of triglycerides is between 50 and 110mg/dl, chylomicron detection on the lipid profile confirms diagnosis. CHT should be differentiated from pseudochylothorax, which produces long-progressing PEs, with cholesterol levels in the PF over 200mg/dl.1
Although no consensus or evidence-based treatment guidelines for CHT are available, initial treatment does not seem controversial and involves decompression of the pleural space and thoracic lymph ducts by continuous drainage through a chest tube. This drainage is more effective than thoracentesis, because the pleural surface in contact with the fistulous area may speed up closure, and continuous drainage also provides accurate monitoring of chyle leak.
A combination of the various treatment options should be applied in a logical order, based on the etiology (congenital, traumatic, malignant, post-surgical, etc.), nutritional and metabolic status, and the absence of surgical contraindications. The underlying disease will be always treated when possible (Fig. 7).58
Nutritional TreatmentThis is based on two basic options to reduce the chyle leak and lower triglycerides. (1) Oral or enteral nutrition avoiding long-chain triglycerides to prevent the formation of chylomicrons, allowing intake of medium chain triglycerides, as these are directly absorbed into the portal system and decrease the flow through the thoracic duct. (2) Absolute bowel rest and parenteral nutrition. Both alternatives have similar results, although the parenteral nutrition options are slightly better.59
Instrumental TreatmentTherapeutic thoracentesis may be sufficient in some cases until treatment of the underlying disease takes effect. If insufficient, a chest tube may be placed for decompression and control of daily leak. Chemical pleurodesis is often performed on patients who do not respond to diet and thoracic drainage. This includes talc instillation through the drainage tube or talc insufflation during thoracoscopy; other forms of mechanical pleurodesis can be performed with thoracotomy or thoracoscopy. Pleurodesis is a well-accepted option for malignant and refractory CHT or when thoracic duct ligation cannot be performed.
PharmacotherapyThis involves the use of a somatostatin analog, octreotide, which decreases splanchnic, hepatic and portal blood flow and also the lymph volume.
Surgical TreatmentThis is based on thoracic duct ligation, usually performed by video-assisted thoracoscopy or thoracotomy at the level of the aortic hiatus. Ligation is the standard surgery for traumatic and iatrogenic CHT in the case of a persistent large chyle leak, malnutrition or immunosuppression, prolonged hospitalization or failure of conservative treatment. Other less frequent treatments include pleuroperitoneal shunt, fluoroscopy-guided percutaneous embolization and anastomosis of the thoracic duct to the venous system.
HemothoraxHemothorax is the presence of blood in the pleural cavity. A bloody PE is considered hemothorax if its hematocrit exceeds 50% of that in peripheral blood.
DiagnosisSymptoms and signs vary depending on the cause, the volume and pace of accumulation. Among traumatic hemothoraces, those secondary to hemodynamic instability are usually more frequent and they are painful, while non-traumatic hemothoraces present with dyspnea and signs of occupation by pleural fluid. Radiography, ultrasound and chest CT reveal the presence of free or loculated PE, occasionally with images compatible with clots that may also reveal the associated lesions that point toward the cause. Definitive diagnosis is obtained by thoracentesis and PF study.
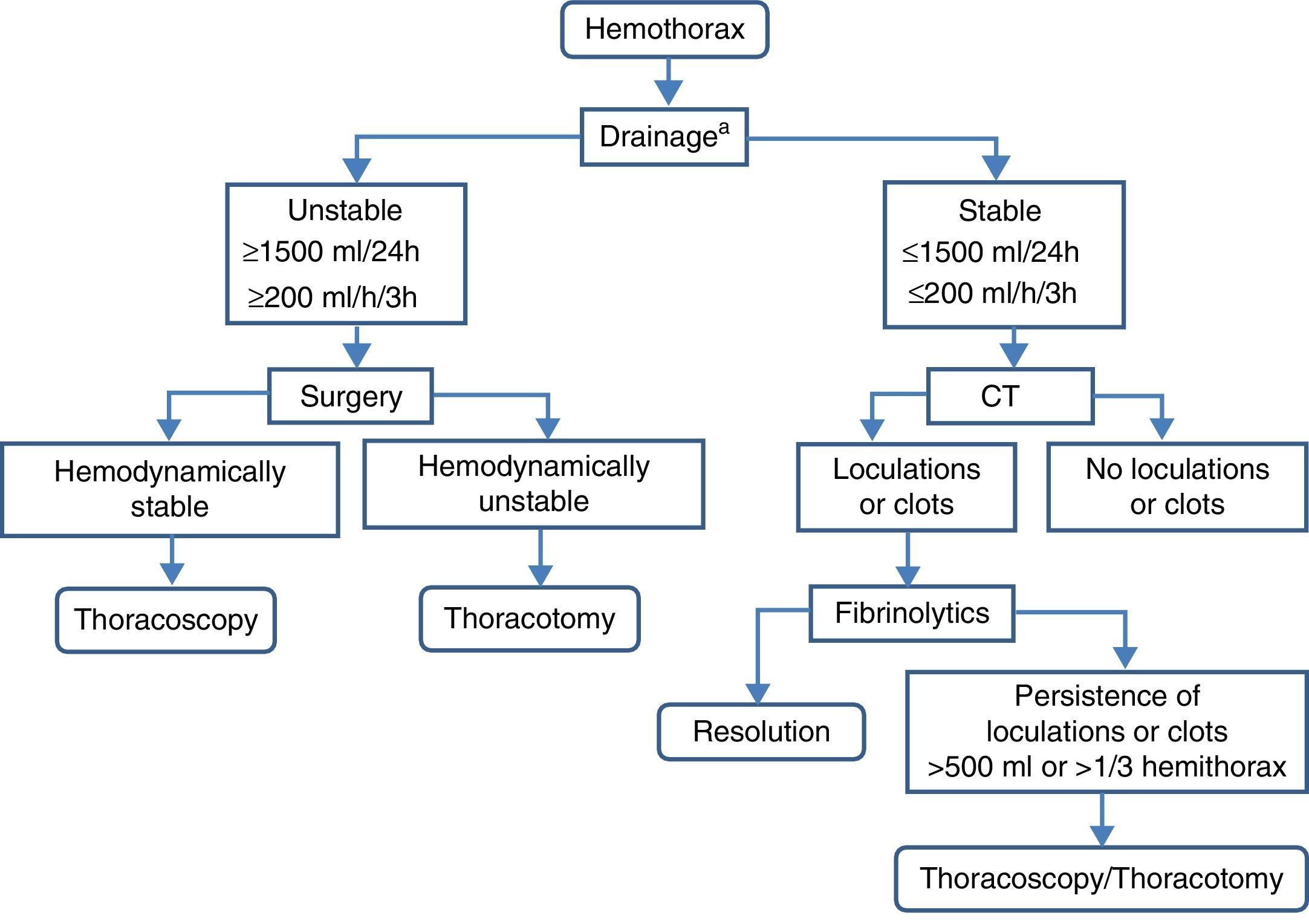
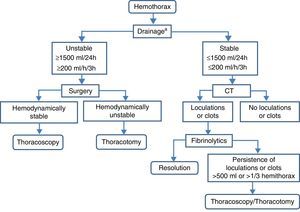
TreatmentAcute and residual hemothorax should be differentiated (Fig. 8).
Acute HemothoraxIn hemodynamically stable patients with small hemothorax (restricted to blunting of the costophrenic angle or calculated volume less than 300ml), clinical–radiological monitoring can be considered (B). An alternative in patients who continue with active hemothorax is video-assisted thoracoscopy, as it usually allows hemostatic actions.60 In hemodynamically unstable patients or when calculated volume is greater than 300ml, a chest tube (gauge 24F or 28F) should be implanted in the mid-axillary line at the sixth intercostal space and directed backwards, after administration of prophylactic antibiotics (B). If the initial drainage is greater than 1500ml/24h (20ml/kg body weight) or the drainage rate is greater than 200ml/h (3ml/kg body weight) for more than three consecutive hours, thoracotomy is indicated (B).
If the hemothorax is suspected to be secondary to aortic aneurysm rupture, drainage is not indicated, because it favors exsanguination (C).
Residual or coagulated hemothorax. Small residual hemothoraces (blunting of the costophrenic angle) may be conservatively treated with physical therapy and respiratory monitoring (B).
Hemothoraces estimated to be over 500ml in volume or with residual loculations or clots occupying a third or more of the hemithorax require treatment to prevent subacute (atelectasis, empyema, pneumonia) or chronic complications (fibrothorax) (B). Implantation of chest tubes can be attempted in the first week. If ineffective, instillation of intrapleural fibrinolytics may be administered (urokinase) (B). Another alternative, or if fibrinolytics are not effective, is early evacuation of clots by thoracoscopy (B).60 If thoracoscopy is not effective, or the hemothorax is chronic and traps the lung (fibrothorax), decortication should be performed (B).
Conflicts of InterestThe authors declare no conflicts of interest in relation to their participation in the SEPAR guidelines on pleural effusion.
| A. Consistent recommendation, high quality evidence. |
| B. Consistent recommendation moderate quality of evidence. |
| C. Consistent recommendation, low quality of evidence. |
| D. Consistent recommendation, very low quality of evidence. |
| E. Weak recommendation, high quality evidence. |
| F. weak recommendation, moderate quality of evidence. |
| G. Weak recommendation, low quality of evidence. |
| H. Weak recommendation, very low quality of evidence. Source: Schünemann et al.2 |
Please cite this article as: Villena Garrido V, Cases Viedma E, Fernández Villar A, de Pablo Gafas A, Pérez Rodríguez E, Porcel Pérez JM, et al. Normativa sobre el diagnóstico y tratamiento del derrame pleural. Actualización. Arch Bronconeumol. 2014;50:235–249.