The great American poet Maya Angelou once said, “In diversity, there is beauty, and there is strength. Diversity makes for a rich tapestry, and all the threads of that tapestry are equal in value no matter their color.”
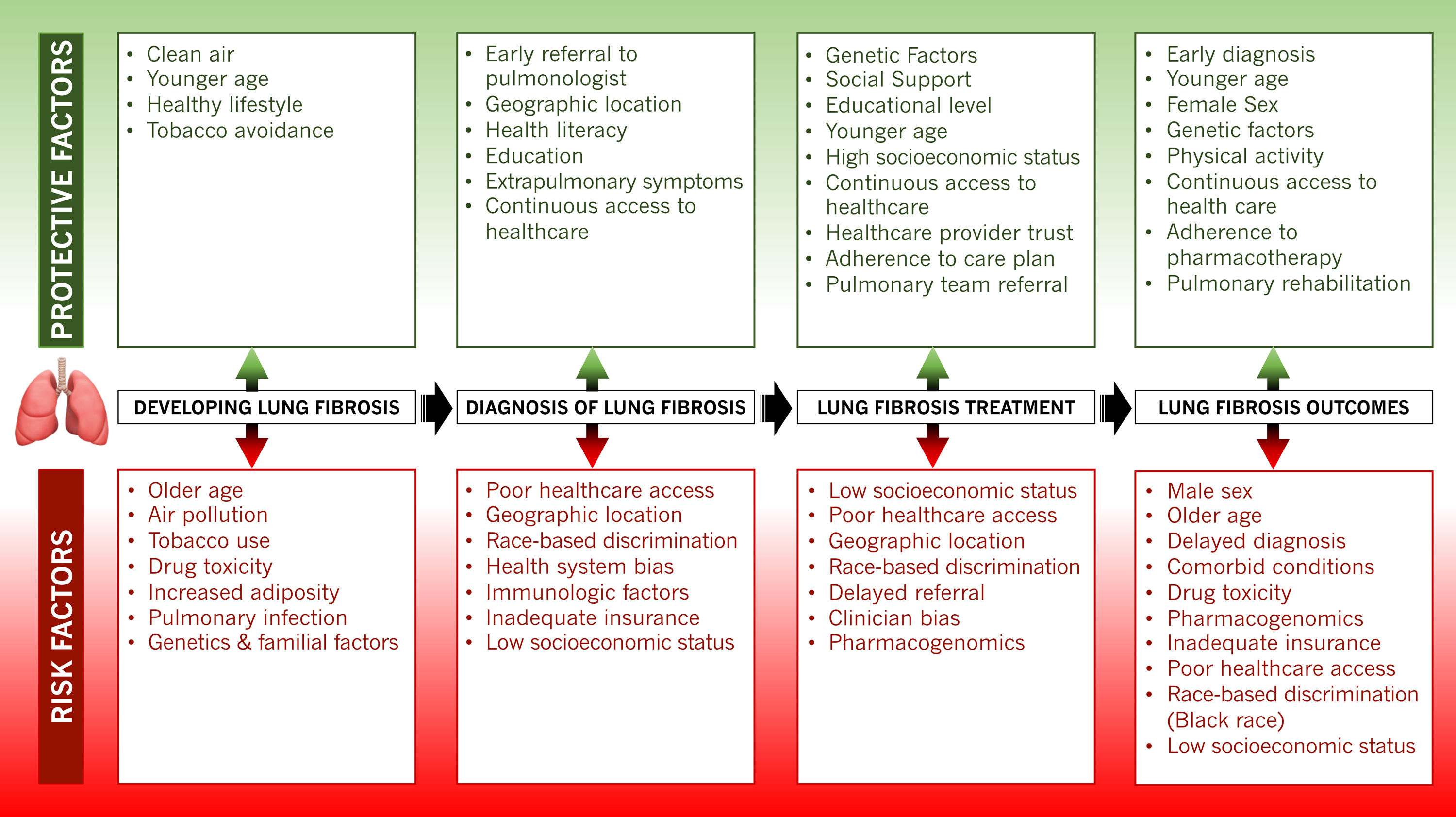
As the racial and ethnic diversity of the world's societies increase, characterizing individual groups based on skin color has become even more complicated and frequently misguided. Repeatedly, this approach carries far-reaching unfortunate ramifications on health outcomes and life expectancy, especially in Black individuals. The complex sociobiological construct that constitutes race reflects an individual's perception of themselves, their cultural background, sociopolitical environment, familial roots, and genetic makeup. However, this perception is often divergent from the individual's true genetic ancestry, a more precise biological entity. The systematic evaluation for disease risk at the point of care often relies on assessing patients for predefined categories to improve efficiency in the diagnostic and treatment process. In these cases, conflating race with biological risk can lead to biased assumptions with detrimental effects on Black populations. In many cases, Black populations’ increased risk for disease and poorer health outcomes is due to social risk factors both within the health care system and in the larger society. Black populations are still impacted by discriminatory policies and practices that have led to stark social and economic disparities that also lead to racial disparities in health. These stark differences are portrayed in the diagnostic modalities for pulmonary fibrosis in Black patients and the subsequent management of their disease (Fig. 1).
Pulmonary fibrosis diagnosis hinges on accurate recognition of hypoxia from pulmonary gas exchange abnormalities, lung volume restriction measured on spirometry, and radiologic indices of lung fibrosis identified on high-resolution chest computed tomography (CT) scans.1 Vital signs performed at every patient encounter routinely include pulse oximetry as a surrogate but reliable index of blood oxygen levels. The precision, ease of use, and affordability of these portable devices have fostered their widespread use beyond clinical settings to now include the home environment, where they often prompt decisions to seek emergency medical care. However, current pulse oximetry algorithms carry substantial bias based on skin pigmentation, and true values for oxygen saturation are often up to eight percent lower than the recorded measurements.2 These errors, which are more profound at lower oxygen levels, are generally greatest in Black subjects. Recent evidence shows that with pulse oximetry, Black patients are almost three times as likely to experience occult hypoxemia as White patients.3 This bias frequently transcends diagnosis to impact clinical decision-making processes such as determining the need for supplemental oxygen and referral for advanced therapies.
Evidence of impaired lung function on spirometric evaluation remains a fundamental requirement for the diagnosis and management of pulmonary fibrosis. The practice of “race adjustment” of spirometric values, which has been mainstream for over half a century, has led to the acceptance of lower lung function measurements in Black individuals as being normal.4 As a diagnosis of respiratory impairment relies on recognizing abnormal values, pulmonary disease in its early stages remains concealed, and injuries perpetuated by any underlying causes continue unabated. Often, this proceeds until severe extrapulmonary manifestations result or lung function impairment exceeds the set thresholds for ‘normal’ in Black individuals.
Black patients are generally less likely to receive diagnostic medical imaging, especially CT scans, compared to other racial and ethnic groups.5,6 This disparity persists even after controlling for other patient and hospital characteristics. In resource-poor settings and developing countries, limited access to chest CT imaging and spirometry is a common impediment to the early diagnosis of pulmonary fibrosis.7 Structural inequities in the healthcare system as well as implicit bias ultimately impacts the medical care received by Black individuals and delays time to diagnosis of pulmonary fibrosis. Even in more developed countries, Black individuals may be uninsured or underinsured and lack access high-quality primary care precluding their ability to obtain diagnostic tests or be referred for subspecialty pulmonology care.8 This further leads to a prolongation in the time to pulmonology referral, a critical determinant of the delay in diagnosis.9,10 These delays can be of grave consequences as the extent of pulmonary fibrosis has been shown to increase by about two percent for each year that the diagnosis remains delayed and is associated with higher mortality.9,11
Inevitably, delayed recognition of the profound alterations in lung mechanics leads to worsened symptoms and quality of life. In addition, the unabated progression of the underlying pathophysiologic processes may impact extrapulmonary organs. This multiorgan involvement results in systemic manifestations that eventually draw attention to the lungs during clinical evaluation. At this point, the urgent need for pulmonary intervention belatedly prompts a medical response. This conundrum might also explain why Blacks in the U.S. and Europe are more likely than Whites to be diagnosed with autoimmune-related causes of pulmonary fibrosis.12,13 In addition, fibrotic sequelae of pulmonary infections such as COVID-19 pneumonitis that disproportionately affects Black individuals could conceivably accentuate these racial disparities and poor outcomes.14
Black individuals also have reduced access to treatment options for pulmonary fibrosis. Over the last decade several antifibrotics have emerged that have been shown to reduce disease progression, respiratory-related hospitalizations, and may improve survival.15 As newer drugs become available to manage pulmonary fibrosis, disparities may widen due to lower rates of trial enrollment, lower physician prescribing and difficulty obtaining high-cost medications.16 Unfortunately, there is as yet no cure for pulmonary fibrosis beyond lung transplantation. However, the same issues of systemic inequities plague access to lung transplantation leading to poorer lung function and worse survival in Black patients even after listing for lung transplant.17
Increasing evidence suggests that racial differences in lung function are less attributable to true innate biological differences but are due more to structural causes of poor health, socioeconomic factors, race-based algorithms, and technological innovations. These fundamental causes need to be addressed. The importance of adequate health insurance coverage and improved access to high-quality primary and subspecialty pulmonology care cannot be overemphasized. Provision of these resources nearly eliminates the health care coverage gap that exists between Black and White populations.18 Federally mandated support for minority populations and community-led strategies that surmount costly pharmacotherapeutics and improves access to lung transplantation are essential steps.
Eliminating these health disparities in pulmonary fibrosis diagnosis and outcomes is attainable and requires all hands on deck. Any meaningful progress in eroding these structural causes of poor health requires an awakening of societal consciousness. We need to understand better the real damage caused to the health of diverse populations from these perceived innate differences and prepare to abrogate them. Public enlightenment campaigns and contemporary informational channels can help refocus the spotlight on harmful belief systems in medicine and society. While governmental policies and industry-led solutions to remediating flawed technological innovations are helpful and necessary, true change also depends on the foundational building block of all human communities – the individual.
FundingNIH K23HL146942.
Author's contributionsConception and design, acquisition of data for the work, analysis and interpretation, and drafting the manuscript for important intellectual content: all authors (AA, MV, MS).
Conflict of interestAA is supported by a career development award from the National Heart, Lung, and Blood Institute (K23HL146942), and has received speaking and advisory board fees from Boehringer Ingelheim and grant funding for interstitial lung disease research from the Pulmonary Fibrosis Foundation. MV, MS have no relevant disclosures.