Lung cancer (LC) is a highly prevalent disease and the leading cause of cancer death worldwide. Although gastrointestinal involvement is very rare, the most common site of metastasis is the esophagus, followed by the jejunum, ileum, stomach, and colon.1 We report the case of a patient with a pulmonary nodule and gastric thickening.
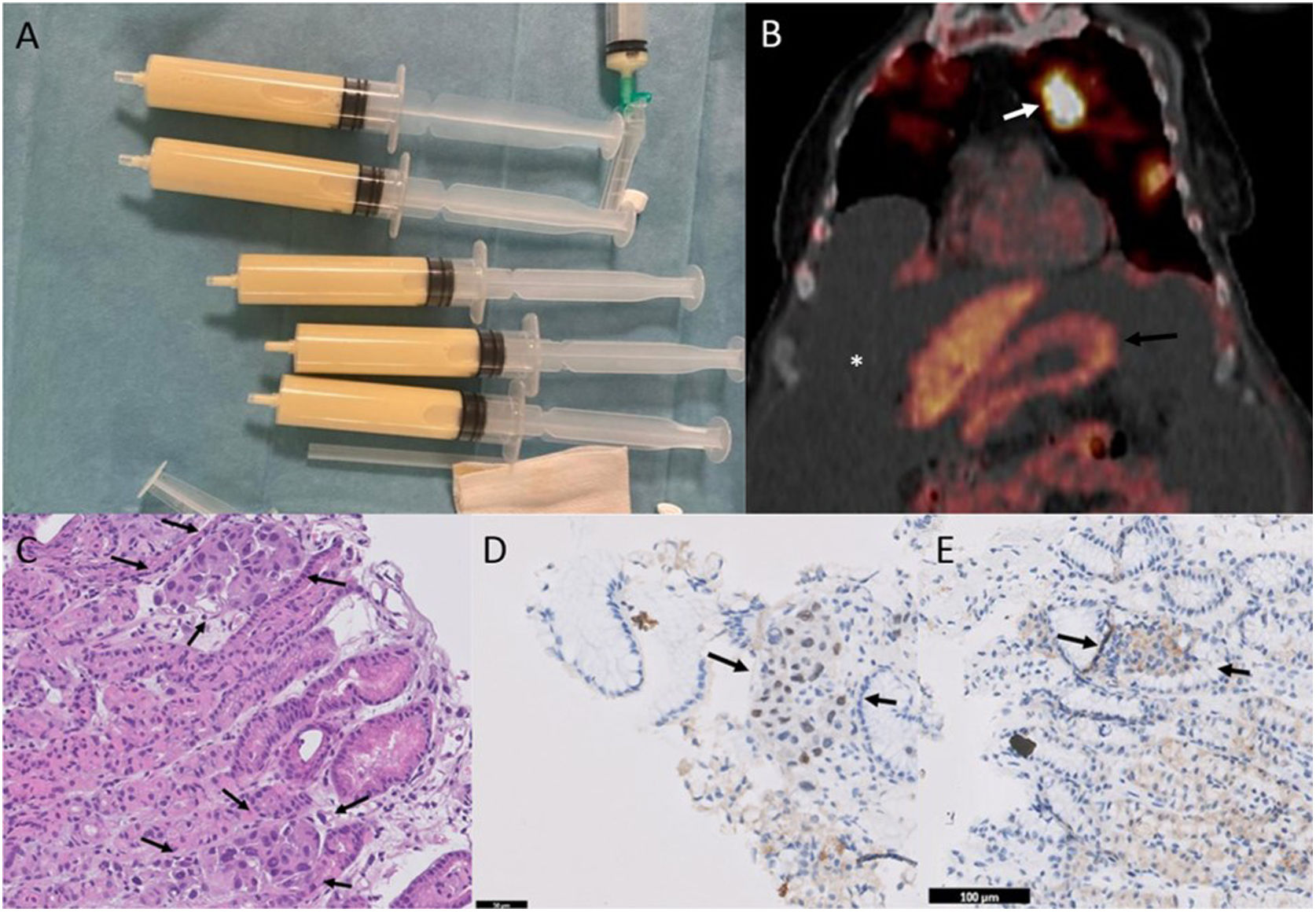
Our patient was an 81-year-old woman whose only personal history of interest was passive smoking. She was admitted for dyspnea, asthenia, and increased abdominal girth. Chest X-ray showed significant bilateral pleural effusion. Thoracentesis was performed, yielding milky pleural fluid consistent with chylothorax (Fig. 1A). Pleural fluid was positive for malignancy with infiltration by adenocarcinoma. Molecular analysis was significant for phenotype BerEP4+/MOC31+/TTF1+, EGFR+ for exon 19 deletion, ALK−, ROS1− and PD-L1−. All these results suggested a primary LC.
(A) Milky pleural effusion consistent with chylothorax. (B) Coronal PET/CT image, revealing increased metabolism in the pulmonary nodule of the left upper lobe (white arrow), moderate–severe ascites (asterisk), and lack of increased metabolism at the gastric level (black arrow). (C) Gastric fundus biopsy (40×, hematoxylin–eosin), showing intact oxyntic gastric mucosa. At higher magnifications, small tumor cell groups are observed, predominantly within the capillaries, with distorted glandular architecture and atypical cells with enlarged nuclei of irregular size. (D) Immunohistochemistry for TTF1 (40×), showing nuclear positivity in tumor cell clusters, consistent with pulmonary origin. (E) Immunohistochemistry for CDX2 (40×), a marker of gastrointestinal differentiation, which is negative in the tumor cell clusters.
A computed tomography (CT) scan was performed that showed a 2.5cm pulmonary nodule in the left upper lobe consistent with a primary carcinoma. The CT also showed non-specific gastric thickening, significant pleural effusion, and ascites. Positron emission tomography (PET)-CT revealed increased metabolism in the pulmonary nodule, with no focal gastrointestinal metabolic changes (Fig. 1B).
Although PET-CT showed no increased metabolism in the gastric region, the possibility of two primary tumors prompted us to perform a panendoscopy. Thickened gastric folds of erythematous appearance were observed in the fundus and the area was biopsied. Pathology results were consistent with infiltration by adenocarcinoma (Fig. 1C). An immunohistochemical study performed to determine the origin revealed TTF1+ and CDX2−, consistent with the primary LC (Fig. 1D and E). The patient was finally discharged with osimertinib treatment and she maintains a partial response at the time of writing.
The appearance of two synchronous tumors is uncommon – the incidence of synchronous or metachronous LC with gastric cancer is 0.4%.2 Similarly, the presence of gastrointestinal metastases from LC has an incidence of only 0.3–1.7%. Gastrointestinal metastasis has very poor prognosis with an average survival of between 1 and 6 months.1
Gastric thickening on CT has been described in desmoplastic reactions, lesional and/or perilesional edema, acid peptic disease, and leiomyomas, which may result in false-positive misinterpretations.3 Therefore, not all gastric thickening on CT is evidence of neoplastic disease, so an endoscopic exploration is imperative.4
PET-CT is also useful in the diagnosis of primary LC metastases and asymptomatic gastrointestinal metastases.1 However, our case confirms that the absence of increased metabolism on PET-CT does not rule out malignant disease. Studies are needed to clarify the sensitivity and specificity of this procedure in the detection of gastrointestinal metastases.
FundingThis paper has not received any funding.
Conflict of interestsThe authors state that they have no conflict of interests.