To the Editor: Carbon monoxide (CO) intoxication is a common occurrence in industrialized cities. Most intoxications are accidental. CO is a product of the imperfect combustion of hydrocarbons, and the amount of gas absorbed depends on minute ventilation, time of exposure, and relative concentrations of CO and oxygen in the atmosphere. We report the case of a woman who developed pulmonary infiltrates secondary to carbon monoxide intoxication.
A 25-year-old woman with no relevant medical history, drug allergy, or toxic habits, and treated with oral contraceptives, came to the emergency service of our hospital complaining of dyspnea, malaise, somnolence, dizziness, and reporting an episode of loss of consciousness without relaxation of sphincters or convulsions. Symptoms had begun the night before admission.
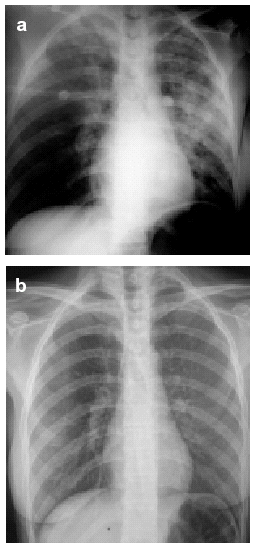
On physical examination, the patient was afebrile, blood pressure was 110/55 mm Hg, and heart rate was 125 beats/min. On auscultation of the lungs, vesicular sounds were diminished on both sides. A chest x-ray showed an increase in alveolar and interstitial density in the right and left upper lobes and left lower lobe (Figure 1a). Blood tests showed a white cell count of 25 000 cells/mL with 85% concentration of polymorphonuclear cells; other parameters were well within the normal ranges. The baseline arterial blood gas analysis showed pH to be 7.22, PaO2 44 mm Hg, PaCO2 29.7 mm Hg, and bicarbonate concentration 12.5 mmol/L. Carboxyhemoglobin was 23.6% and lactic acid was 2.16 mmol/L.
Figure 1. Posteroanterior chest x-rays showing: a) the pulmonary infiltrates toward the onset of symptoms and b) the resolution of the infiltrates within a few hours.
The patient was diagnosed with CO intoxication and suspected aspiration pneumonia, and highly concentrated oxygen therapy was prescribed along with sodium bicarbonate, corticosteroids, antibiotics, and bronchodilators. The only source of CO the patient had been exposed to was a home boiler. The clinical response was satisfactory. The chest x-ray at 24 hours of admission was normal and pulmonary infiltrates disappeared (Figure 1b). Two days after admission, respiratory function tests were carried out: vital capacity was 3.88 L (96%), forced vital capacity (FVC) was 3.62 L (91%), forced expiratory volume in the first second (FEV1) was 3.26 L/s (93%), and the ratio FEV1/FVC was 90%. The CO diffusion capacity was 8.23 mmol/min/KPa (82.3%) and the diffusion per unit of lung volume (KCO) was 1.66 mmol/min/KPa/L (77.3%). KCO change was monitored for 1 year and was observed to increase.
CO is an odorless, colorless, nonirritating gas that is easily absorbed by the lungs and whose toxicity is due to the combination of tissue hypoxia and direct cell damage.1 CO competes with oxygen to combine with hemoglobin, for which it has a 200- to 500-fold higher affinity.2 The consequence of carboxyhemoglobin combination is a reduction in the amount of oxygen bound to hemoglobin and a shift in the oxygen-hemoglobin dissociation curve to the left, so that the availability of oxygen in tissues decreases. The tissues that are most sensitive to the toxic effect of this gas are those with greater metabolic need, such as the central nervous system and the myocardium. The most frequent symptoms are headache, dizziness, asthenia, nausea, vomiting, and difficulty concentrating.3
A high level of suspicion is necessary to reach a diagnosis of CO intoxication. A firm diagnosis is established by measuring blood carboxyhemoglobin values,4 which are high in these patients, normal values being 5% to 9% in adult smokers and 2% in adult nonsmokers. Decreased oxygen saturation, altered PaCO2 and PaO2, and metabolic acidosis caused by an anaerobic metabolism in hypoxic tissues are evident in arterial blood gas measurements.5
Pulmonary infiltrates have been described in patients who have been in burning buildings and have inhaled CO and other gases resulting from the combustion of plastics and other synthetic materials that irritate the respiratory mucosa. In CO intoxication, pulmonary involvement has been described only rarely. In a series of 2759 patients, 35 had pneumonia and 10 had pulmonary edema, though the latter was observed in more than a half of the most serious cases. Two pathogenic mechanisms related to hypoxia have been suggested: altered permeability of lung capillaries and myocardial infarction.6
In the case we present, the patient had only been exposed to CO. The presence of pulmonary infiltrates was first attributed to aspiration pneumonia occurring when she was unconscious, and antibiotics were therefore prescribed. However, as the patient never developed fever and the infiltrates disappeared in a few hours, that diagnosis was unlikely. The parenchymal changes were attributed to the possible presence of noncardiogenic pulmonary edema caused by CO, although we cannot rule out the possibility of chemical pneumonitis secondary to the aspiration of gastric content.
Oxygen (100%) is administered to treat CO intoxication in order to shorten the half-life of carboxyhemoglobin and increase oxygen uptake by tissues. Comatose patients may require intubation and mechanical ventilation, and hyperbaric oxygen may be useful, though it is controversial. However, some authors believe that hyperbaric oxygen reduces late functional abnormalities related to the use of normobaric oxygen, even in noncomatose patients.