The term ‘colonization,’ when applied to the bacterial presence in or on the human body, implies that it is not harmful to the host in order to distinguish it from ‘infection.’ There is now ample evidence that acquisition and persistence of certain bacterial pathogens (Haemophilus influenzae, Moraxellacatarrhalis, and Streptococcus pneumoniae) in the lower airways in all stages of chronic obstructive pulmonary disease (COPD), with or without a change in baseline respiratory symptoms, is harmful to the host. Pseudomonas aeruginosa (PA) acquisition and colonization is usually found in advanced stages of COPD.1 This raises the important question, whether PA colonization is a consequence of advanced COPD, i.e., the chicken, or is it contributing to COPD pathogenesis, i.e., the egg.
Epidemiology of PA in COPDPA isolation from sputum has been reported in 4%–15% of COPD patients in older cross-sectional studies.2 In several studies, the prevalence of PA colonization increases significantly with increasing severity of underlying airflow obstruction. Engler et al. reported that isolation of PA increased from 0.7% to 2.6% in mild to very severe COPD.3 Another recent epidemiological study of 22,053 COPD patients showed sputum isolation of PA in 4.2% during a median follow-up of 36 months.4
The patterns of colonization in COPD are distinct from Cystic Fibrosis (CF), in which eventually almost all patients develop persistent colonization with mucoid PA strains.5 In a longitudinal study in mild to very severe COPD with monthly sputum sampling, of the 57 new acquisitions of PA, 16 cleared within one month, 15 cleared after 2 to 22 months, and 13 isolates never cleared. In another cohort of 118 severe COPD patients with monthly sputum sampling, 34.7% had isolation of PA; however, only 12.2% had chronic colonization.6 In another cohort of 170 patients with moderate to very severe COPD sampled every 3–6 months, 24.1% of patients had isolation of PA, and 14.1% had chronic colonization.7 Clearly, colonization by PA in COPD can be transient or persistent, and the patterns may have different clinical implications.
In addition to the severity of airflow obstruction, risk factors for PA isolation in COPD include multiple courses of systemic antibiotics or steroids, high dose inhaled corticosteroids, bronchiectasis, a previous intensive care unit stay, current smoking habit, and previous isolation of PA.8,9 Risk factors for chronic colonization by PA in COPD include previous severe exacerbations,7 increasing severity of COPD, multi-drug resistant strains,10 prior antibiotic use, and repeated exposure to systemic steroids.7 Whether bronchiectasis on CT is a risk factor for chronic colonization is debated as studies have shown contradictory results.6,7
Adaptation by PA to the Airway Milieu in COPDMartinez-Solano et al. reported a greater mutation rate, antibiotic resistance and biofilm production, and reduced protease production, cytotoxicity, and motility in sputum PA isolates from COPD patients. These changes were similar to those seen in persistent sputum isolates in CF.11
Although mucoid strains play a prominent role in CF, only a small number of strains of PA isolated from COPD patients are mucoid.3,5 The proportion of mucoid strains has varied from 8.5 to 14.1% in recent studies in COPD, and not all the mucoid strains were persistent.3,5,6 Mucoid strains in COPD were usually seen in advanced disease and with significant radiological bronchiectasis.3,6
Similar to CF, a significant modification in gene expression in PA sputum isolates that facilitates persistence has been shown in COPD. Rakhimova et al. reported that the PA isolates from COPD patients showed an underrepresentation of exoU-positive strains.12 ExoU is a potent cytotoxin and can cause lung tissue destruction, suppression of which would facilitate chronic bacterial colonization. Another observation was that the distribution of FpvA receptors (Pyoverdines) differed by the duration of colonization in COPD. Sporadic isolates have type 1 FpvA overrepresentation, and persistent isolates showed an increased frequency of type IIb and type III FpvA receptors.12 The pKL-3 gene, a marker for genome mobility and horizontal gene transfer, also showed a significant association with the duration of PA colonization in COPD.12
Clinical Outcomes Associated With PA in COPDIn COPD, approximately 40% of new acquisitions were associated with an acute exacerbation. The risk of exacerbation is increased 3.36 fold (RR 1.88–6.03, 95% CI) with the acquisition of a PA strain new to the patient.5
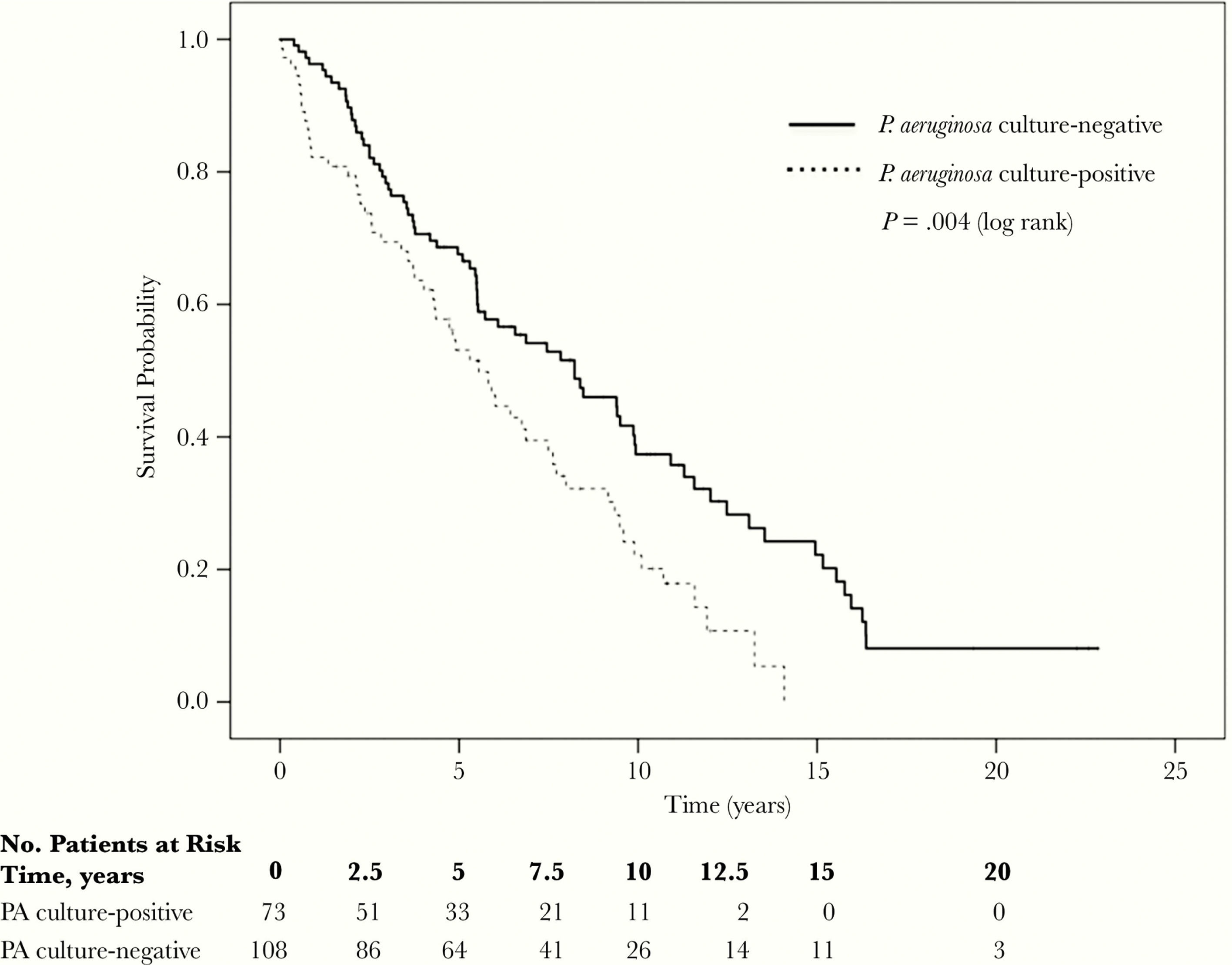
Several recent investigations have described adverse long-term clinical outcomes with PA colonization in COPD. These include increased exacerbation rates, prolonged hospital stay, and increased all-cause mortality.4,6,7 One study reported a doubling of exacerbations requiring hospitalization after the first PA colonization.4 Jacobs et al. reported that both the first isolation and multiple isolations of PA were linked to a 47% higher mortality risk and higher hospitalization rates2 (Fig. 1). However, two studies reported that the first and single PA isolation did not increase all-cause mortality,7,13 but PA persistence did.7
Kaplan–Meier curves comparing overall survival in a longitudinal cohort study for the Pseudomonas aeruginosa (PA) culture-positive and culture-negative groups.2
PA-specific treatment needs to be considered in COPD in the treatment of acute exacerbations, prevention of exacerbations, and prevention of persistent colonization after acquisition. Unfortunately, there is a paucity of good quality clinical data in all three; therefore, the approach to treating PA in COPD is largely based on expert opinion and preclinical data.
Sputum culture results are not available at the time of an exacerbation; therefore, the decision to target PA with empiric antibiotics is often based on risk factors for PA infection, as described above. In outpatients, the presence of such risk factors would prompt the use of an oral antipseudomonal antibiotic, such as ciprofloxacin. Among inpatients, parenteral antibiotics are used for PA, most commonly a βlactam/lactamase inhibitor such as Piperacillin/Tazobactam is a reasonable choice.14 Clinical trial data to support these recommendations is lacking. In fact, a prospective clinical trial in 170 patients with COPD exacerbations requiring mechanical ventilation comparing Ciprofloxacin to Trimethoprim-sulfamethoxazole found no difference in outcomes. However, the number of PA isolates in this study was small (n=13).15 A recently published multicenter retrospective cohort study in hospitalized patients compared anti-pseudomonal with non-anti-pseudomonal antibiotics found no difference in hospital length of stay.16 However, the proportion with PA isolation in both groups was small.
Prevention of Exacerbations and ColonizationInhaled AntibioticsInhaled antibiotics active against gram negative pathogens, including PA, reduce exacerbations in CF and Bronchiectasis. There are no randomized controlled trials to show such efficacy in COPD, although there is encouraging data from small open label cohort studies. Dal Negro et al. treated 13 severe COPD patients colonized by multi-drug resistant PA with nebulized tobramycin for 14 days and found a reduction in local inflammation, bacterial load, and severe exacerbations during a 6-month follow-up.17 Bruguera-Avila et al. used nebulized colistin for three months in 24 severe COPD patients with recurrent exacerbations and noted decreased hospitalization, reduced length of hospital stay, and 40% eradication rates, although there was no difference in the number of outpatient exacerbations.18
Recently, a multicenter observational study examined the effectiveness and safety of inhaled antibiotics in 693 COPD patients with multiple exacerbations and bacterial colonization with any potentially pathogenic microorganisms. They reported a significant decrease in the number of exacerbations, hospital admissions, and length of hospital days, with positive patient outcomes more pronounced in PA eradicated patients.19 Monton et al. used nebulized colistin for three months+cyclic Azithromycin in 32 severe COPD patients with recurrent exacerbations showed a significant reduction in the number of COPD exacerbations from other causes and by PA. PA eradication rates of 28% were noted over two years.20 Though these studies are encouraging, the evidence is not strong enough to recommend inhaled antibiotics in all PA colonized COPD patients, though such therapy could be considered on an individual basis.
Systemic Antipseudomonal AntibioticsPrevention of persistence by early aggressive antibiotic therapy when PA is acquired is often practiced in children with CF, however no studies to support this practice in COPD, though at least open-labeled RCT is ongoing (NCT03262142).
Definitive clinical trials for PA treatment or prevention in COPD face significant challenges, including the high rate of transient colonization, the relatively small proportion of patients who ultimately acquire PA, and the lack of sensitivity of sputum cultures for PA colonization in COPD.5
Summary and Future NeedsIn summary, PA does appear to be an ‘egg’ rather than a ‘chicken’ in COPD, with mounting evidence of its role in exacerbations, hospitalizations, and mortality. Though only 5% of COPD patients appear to be infected by PA because COPD is so prevalent, the absolute number of such patients is quite large, and their care consumes considerable health care resources. There is a desperate need for studies that will inform us how to best manage these patients, including appropriate acute and chronic antibiotic therapy, the role of anti-inflammatory and immunomodulatory medications, as well as of airway clearance techniques. We also need to further develop novel molecular diagnostic techniques and biomarkers to better characterize PA epidemiology and impact in COPD.
Financial SupportNone.
Conflicts of InterestR. Kunadharaju has no conflicts of interest to disclose.
A. Rudraraju has no conflicts of interest to disclose.
S. Sethi has received research funding (Mylan; Regeneron), speaking honoraria and has been a consultant (BI; Astra Zeneca; GSK; Theravance; Pulmonx; Merck, Nuvaira; Chiesi).