The Spanish COPD guidelines (GesEPOC) have been recently modified. The aim of this study is to assess this revision and evaluate the prognosis of patients according to the new classification of severity.
MethodsA total of 700 COPD patients (83.9% men) were prospectively followed up for a mean period of 5 years in tertiary hospitals in Spain and the USA. Anthropometric data, lung function tests, dyspnea (according to the mMRC scale), BODE and Charlson index were collected. We calculated mortality at 5 years following the risk criteria proposed by the new GesEPOC.
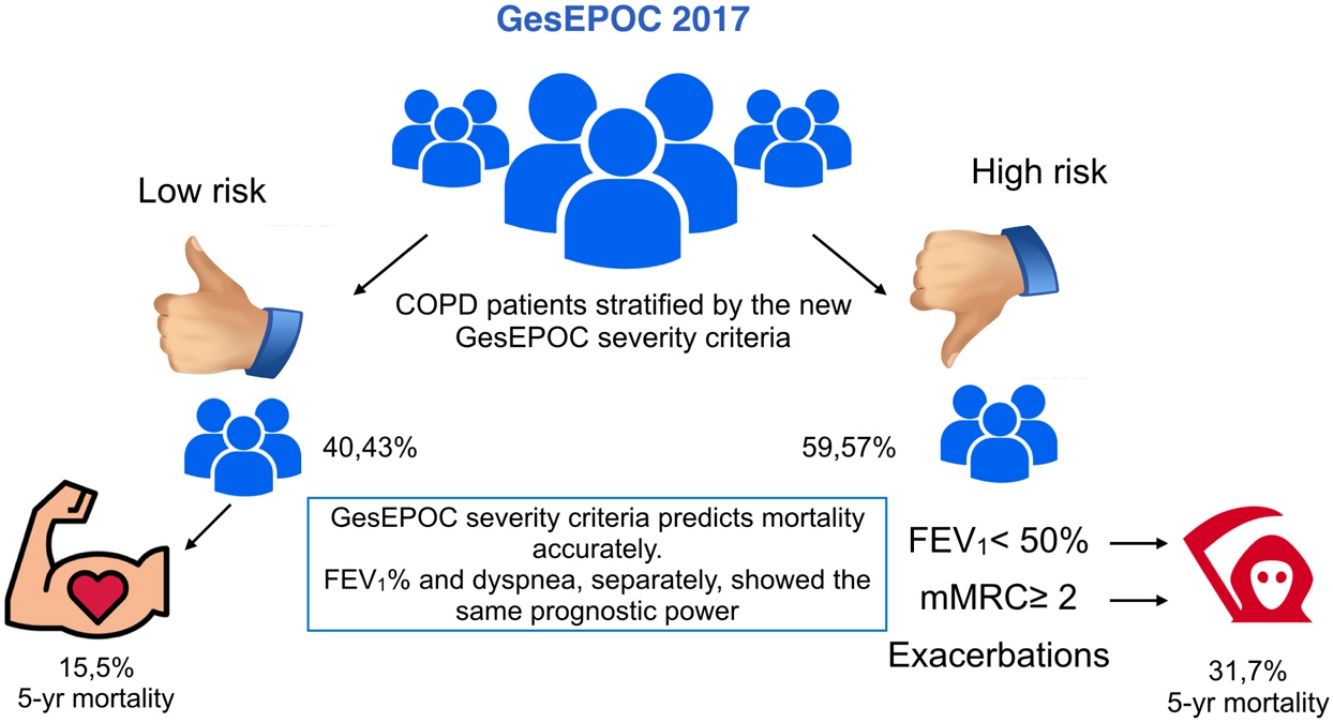
ResultsMean age was 66±9.6 years and mean FEV1% was 59.7±20.2. The proportion of patients in the low-risk group was 40.43%. Patients in the high-risk group had a significantly higher BODE index than those in the low-risk group (2.92±0.66 vs 0.52±1.91, P<0.001), while the Charlson index score was similar in both groups. Mortality at 60 months was significantly higher in the high-risk group (31.7% vs 15.5%, P<0.001). Dyspnea and FEV1% were also independent predictors of mortality (P<0.001), and neither was inferior to the risk classification proposed by GesEPOC.
ConclusionsThe new severity index proposed by GesEPOC accurately predicts 5-year mortality. However, dyspnea and FEV1% have the same strength in predicting mortality.
La Guía española de la EPOC (GesEPOC) ha sido recientemente modificada. El objetivo de este trabajo es valorar la clasificación y el pronóstico de los enfermos según la nueva clasificación de la gravedad.
MétodosSe siguió a 700 enfermos con EPOC (83,9% varones) durante un periodo medio de 5 años en hospitales españoles y de EE. UU. Se midieron datos antropométricos, función pulmonar, disnea medida con la escala mMRC, así como exacerbaciones y los índices de BODE y Charlson. Se clasificaron según el riesgo proporcionado por GesEPOC y se valoró el pronóstico a 5 años.
ResultadosLos pacientes tenían una edad media de 66±9,6 años y un FEV1% de 59,7±20,2. El 40,43% de la muestra se encontraba en bajo riesgo. Los sujetos del grupo de alto riesgo presentaban un índice de BODE significativamente mayor que los de bajo riesgo (2,92±0,66 vs 0,52±1,91, p<0,001). El índice de Charlson fue similar entre ambos grupos. La mortalidad a 60 meses en el grupo de alto riesgo fue significativamente mayor que en el de bajo riesgo (31,7% vs 15.5%, p<0,001). Tanto la disnea como el FEV1% fueron también predictores independientes de mortalidad (p<0,001), siendo cada uno de ellos no inferior prediciendo mortalidad que el conjunto de los criterios del grupo de alto riesgo de GesEPOC.
ConclusionesLa nueva clasificación de la gravedad de GesEPOC predice la mortalidad de forma adecuada. No obstante, tanto el FEV1% como la disnea tienen la misma potencia para predecir mortalidad.
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of morbidity and mortality worldwide,1 and is highly prevalent and underdiagnosed in Spain.2 The first Spanish COPD Guidelines (GesEPOC)3 were published in 2012 as part of the Quality Plan of the National Health System and the COPD strategy of the Ministry of Health, Equality and Social Policy. In the initial version, both patient classification and treatment were defined according to the existing COPD phenotypes. This first version has been recently amended with a substantial change in the approach to these patients. The initial classification by phenotypes has shifted to a classification based on risk, with a low-risk group and a high-risk group.4 The definition of risk is based on lung function, dyspnea, and exacerbations, along the same lines as the Global Initiative for Obstructive Lung Disease (GOLD) document.5 However, the criteria for assigning the different risk groups are different from those proposed by GOLD. The decision was taken to discard the multidimensional severity indices specified in the previous version (BODEx),6 to make way for a simpler, more clinical classification system. Treatment with 1 or 2 bronchodilators is proposed, depending on the patient's risk group. A pharmacological intervention is then designed according to phenotype (with the exception of the mixed phenotype, which would receive inhaled corticosteroids from the outset). This clinical guideline concept will probably encourage primary care physicians to follow the recommendations. However, this new vision of the classification of risk has not yet been evaluated, and we know little about how it affects the distribution of patients and their long-term prognosis.
This prospective study of COPD patients assessed the distribution of the population according to overall risk and the individual components of the classification. It also evaluates the long-term prognosis of patients according to the new GesEPOC classification.
MethodsParticipants in this study are part of the BODE cohort, which consists of COPD patients followed consecutively in respiratory clinics between 1997 and 2017. The inclusion and exclusion criteria have been described elsewhere.7 In summary, this is a longitudinal, prospective, multicenter cohort with at least 1 annual check-up. The anthropometric characteristics of all patients are collected, and a physical examination is performed. A spirometry test following the American Thoracic Society and European Respiratory Society criteria is performed, and COPD is diagnosed according to internationally established criteria.8,9 All patients in the cohort had a pack-year index (PYI) of at least 10 and were stable for a minimum of 6 weeks prior to the tests. Exacerbations are defined as any episode of worsening dyspnea, cough or expectoration that requires antibiotics or systemic corticosteroids.10 Exacerbations were reported by the patient and later confirmed from their electronic medical record. They were classified as moderate if they were ambulatory and treated with oral steroids or antibiotics, or severe if hospitalization was required. Dyspnea was measured using the modified Medical Research Council (mMRC) scale.11 The BODE index and the Charlson index were also calculated.7,12 Comorbidities were collected according to the diseases reported by the patient and from a review of the electronic medical record. The 6-minute walk test (6MWT) was performed, and the best of 2 separate attempts made at least 30min apart was recorded.13 Overall mortality until February 2017 was collected. We also analyzed death due specifically to COPD, respiratory causes not associated with COPD, lung cancer, cardiovascular causes, other causes, and unknown causes. All participants received optimal treatment according to current international guidelines and signed informed consent forms that were previously approved by the ethics committees of each participating center.
GesEPOC 2017Patients were classified as high or low risk following the current GesEPOC guideline criteria (any of the following criteria would lead to the patient being classified as high risk: FEV1% <50%, dyspnea ≥2 if receiving treatment, 2 or more outpatient exacerbations, or at least 1 exacerbation requiring hospitalization during the previous year). We then calculated the proportion of subjects who were classified as high risk according to the individual risk factors that make up the overall criteria, and those subjects who were high risk with more than 1 risk factor. Comorbidity by groups and 5-year mortality according to the different causes were calculated.
StatisticsThe Shapiro–Wilk test was used to establish the normality of the quantitative variables. Mean and standard deviation were calculated for variables with a normal distribution. Variables that do not present a normal distribution are described by the median and the interquartile range (Q1–Q3). Fisher's exact test was used to compare qualitative variables. Logistic regression models were created to predict 60-month mortality among the GesEPOC high-risk model and the 4 variables that comprise it, distinguishing between outpatient and inpatient exacerbations. All models were adjusted for age and gender. ROC curves and their respective areas under the curve (AUC) have been calculated to measure the predictive capacity of these models. Quantitative variables were compared using the Student's t test. We also used the Kaplan–Meier method to graphically represent the survival curves and the log-rank test to compare differences between these curves. A P-value of <0.05 was considered statistically significant. We used the R Core Team (2018) statistical program.14
ResultsStudy population characteristics are shown in Table 1. Seven hundred patients were included with a mean age of 66±9.6 years and an FEV1% of 59.7±20.2. Most patients had moderate or severe obstruction. More than 80% were men and a third of the series were active smokers. The pack-year index was high, with a mean of 60±31.16.
Characteristics of COPD Patient Population.
| N | 700 |
|---|---|
| Men, n (%) | 587(83.98) |
| Age (mean±SD) | 66.4±9.26 |
| BMI (mean±SD) | 27.43±4.9 |
| Active smokers, n (%) | 236(33.76) |
| PYI (mean±SD) | 60.66±31.16 |
| FEV1% (mean±SD) | 59.71±20.21 |
| GOLD1, n (%) | 122(17.43) |
| GOLD2, n (%) | 348(49.71) |
| GOLD3, n (%) | 179(25.57) |
| GOLD4, n (%) | 51(7.29) |
| Charlson index (mean±SD) | 2.25±2.31 |
| BODE index (mean±SD) | 1.93±1.93 |
| Classification according to GOLD, n (%) | |
| A | 373(53.29) |
| B | 184(26.29) |
| C | 60(8.57) |
| D | 83(11.86) |
| Dyspnea (mean±SD)a | 1.34±1.09 |
| Dyspnea <2, n (%) | 524(74.86) |
| Exacerbators, n (%)b | 174(25.63) |
| Inpatient exacerbator, n (%)c | 66(9.43) |
| Outpatient exacerbator, n (%)d | 108(15.43) |
| High risk according to GesEPOC, n (%) | 417(59.57) |
| Death, n (%) | 176(25.14) |
| Pulmonary death, n (%) | 88(12.57) |
| COPD death, n (%) | 51(7.29) |
| Cardiovascular death, n (%) | 22(3.14) |
| Lung cancer death, n (%) | 33(4.71) |
| Follow-up time (mean±SD) (months) | 53.61±34.44 |
BODE: body mass index, airflow obstruction, dyspnea and exercise capacity; BMI, body mass index; GesEPOC: Spanish COPD guidelines; GOLD: Global Initiative for Chronic Obstructive Lung Disease; PYA: pack-years index; SD: standard deviation.
A total of 40.43% of the sample were in the low-risk group. In the high-risk group, 38.14% had dyspnea greater than or equal to 2 on the mMRC scale, and 15.43% had experienced outpatient exacerbations, while 9.43% have just had hospitalary exacerbations (total number of exacerbators: 174, 24.86% of the series). A total of 32.86% of participants had FEV1 <50%. A total of 30.71% of patients classified as high risk only had 1 risk factor, 19.86% had 2 risk factors, while 8.14% and 0.86% had 3 or 4 risk factors, respectively (outpatient and inpatient exacerbations were separated).
Comorbidities and SeverityPatients in the high-risk group had a significantly higher BODE index than those in the low-risk group (2.92±0.66 vs 0.52±1.91, respectively, P<0.001). The BODE index was higher in all subjects who presented any risk factor, irrespective of which one. The Charlson index did not differ between high and low-risk groups (2.38±2.21 vs 2.27±2.26, respectively, P=0.57). There is a marginally significant association between subjects who presented inpatient exacerbations and a higher comorbidity burden (P=0.07).
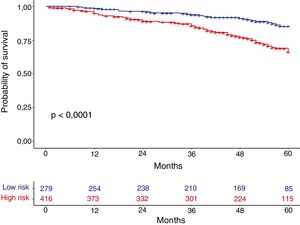
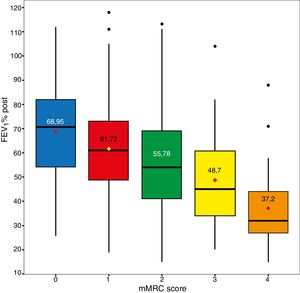
MortalityOverall mortality at 60 months was 25.4%, and was significantly higher in the high-risk than in the low-risk group (31.7% vs 15.5%, respectively; P<0.001) (Fig. 1). Dyspnea, exacerbations requiring hospitalization and FEV1% were all significantly related to mortality (P<0.001, P=0.0012, and P<0.001, respectively). Dyspnea and FEV1% were inversely related with a Spearman index of −0.39 (Fig. 2). Outpatient exacerbations were not related with mortality (P=0.24). Mortality due to cardiovascular causes was not higher in the high-risk subjects than in the low-risk group (3.8% vs 2.1%, respectively; P=0.27). However, pulmonary death was higher in the high-risk group (6.7% in the low-risk group vs 16.5% in the high-risk group, P<0.001), as was COPD death (1.8% in the low-risk group vs 11% in the high-risk group, P<0.001). Cardiovascular mortality was higher in subjects with the FEV1 < 50% risk factor (2.1% vs 5.2% for low and high-risk groups, respectively, P=0.048). Lung cancer mortality was independent of the risk classification. The mortality rate increases linearly with the number of risk factors presented by each patient (P<0.001). This association is shown in Table 2. The prediction of mortality according to GesEPOC risk stratification, as measured by the probabilities obtained using the logistic regression model, was not significantly better than predictions calculated exclusively from dyspnea, FEV1% or exacerbations requiring hospitalization (Fig. 3A and B).
Number of Risk Factors According to the GesEPOC Risk Classification (FEV1%, Dyspnea Measured with the mMRC Scale, Inpatient and Outpatient Exacerbations) and their Relationship with Mortality.
| Number of Risk Factors | Death | Total | |
|---|---|---|---|
| No | Yes | ||
| Low risk, n (%) | 239(84.5) | 44(15.5) | 283(40.4) |
| 1 risk factor, n (%) | 160(74.4) | 55(25.6) | 215(30.7) |
| 2 risk factors, n (%) | 92(66.2) | 47(33.8) | 139(19.9) |
| 3 risk factors, n (%) | 32(56.1) | 25(43.9) | 57(8.1) |
| 4 risk factors, n (%) | 1(16.7) | 5(83.3) | 6(0.9) |
| Total | 254 | 176 | 700 |
GesEPOC: Spanish COPD guidelines; mMRC: modified Medical Research Council.
ROC curves for the different predictive models of mortality and their comparison. (A) Dyspnea vs high-risk group criteria according to GesEPOC; (B) FEV1% vs high-risk group criteria according to GesEPOC; (C) high-risk group criteria according to GesEPOC vs high-risk group criteria considered separately.
In this study, in which COPD patients were followed prospectively and classified according to the criteria recommended by the GesEPOC 2017 guidelines, 40.43% of patients were in the low-risk group, while 59.57% were classified as high risk. The high-risk group, despite having similar comorbidities, had higher mortality than the low-risk group. The elements that define the high-risk group (FEV1, dyspnea, and exacerbations) are independently related to mortality, with the exception of outpatient exacerbations. Data from other studies support some of our results, including mortality and, to a lesser extent, patient distribution.15
The factor most closely associated with mortality is lung function, followed by dyspnea (with no statistical differences between these variables) and exacerbations requiring hospitalization, which have a predictive capacity for mortality similar to that of the set of different criteria that make up the high-risk group. Additional risk factors are associated with a poorer prognosis, with differences being observed among patients who present only 1 variable and those with 2, 3 or 4 variables. This difference in prognosis depending on the number of risk factors is even more marked in patients in the high-risk group, when subdivided according to severity.
The increase in the prevalence of cardiovascular disease in subjects who have dyspnea has previously been described, but in this study, mortality associated with the high-risk group was not due to cardiovascular diseases, but instead to pulmonary disease and COPD. The only factor associated with vascular comorbidity is FEV1%, an observation that has previously been published.16–18 It is therefore unlikely that the prognostic power of dyspnea for mortality is a result of its association with cardiovascular diseases.
The new GesEPOC system for the classification of severity is a departure from the proposals of the previous guidelines. The current approach is simpler, and seems to concur well with prognosis. The inclusion of lung function in the classification of patients with COPD who may need specialized care makes sense. However, it is interesting to note that the prediction of mortality proposed by the guidelines is not superior to dyspnea or FEV1% alone. This is probably due to the linear relationship between these 2 parameters, which are the main components of this classification. This linear relationship is not perfect, but it should not be underestimated.
The power of FEV1% to predict mortality is higher in our study than that of dyspnea. However, the relationship between cardiovascular death and FEV1% may be influencing this difference. Dyspnea remains a powerful and reliable predictor of pulmonary and COPD death. We might ask whether in areas such as primary healthcare, where performing spirometry can be difficult, it would be possible to use dyspnea as a marker of severity and as a major variable for guiding treatment once it has been diagnosed.19
It is interesting to observe how inpatient and outpatient exacerbations, when analyzed together, are not associated with mortality. This phenomenon is probably due to the designation of frequent exacerbator. The relationship between mortality and exacerbations requiring hospitalization is well known,20 but the relationship between mortality and outpatient or moderate exacerbations has not been defined, and to date has not been demonstrated. To strengthen the classification, outpatient exacerbations should be excluded, and only inpatient exacerbations should be considered, although this does not mean that outpatient exacerbations do not need to be treated or prevented, as these impact on quality of life and use of health resources.21,22 We should point out that only 4.29% of patients are in the high-risk group exclusively due to outpatient exacerbations, minimizing the impact of this factor on the classification system. All other exacerbators required hospitalization (severe) or presented outpatient exacerbations with another additional risk factor.
In our sample, if we compare the GesEPOC classification with the GOLD classification, we find a difference of nearly 40% in prevalence among high-risk GOLD groups (C and D) and the high-risk GesEPOC group. If we make this comparison with groups B and D (the ones associated with higher mortality in the GOLD classification),23 the difference is less, although still high at 21%. This difference is probably due to the presence of a group of subjects that would be classified as GOLD group A but who have FEV1 <50%. These discrepancies clearly influence the treatment proposed by both guidelines for patient management; at least 20% more patients would be prescribed dual bronchodilation if the GesEPOC is used, but this proportion would be much higher (more than 50% of the sample) if the patients who are classified as GOLD grades B and C were treated with the recommendations of first choice (a long-acting bronchodilator in group B and a LAMA in group C). From our point of view, the GesEPOC approach more closely reflects reality and clinical practice in our setting. The exclusion of FEV1% from the GOLD system has distorted its clinical utility and reduced its power to predict disease prognosis.
Our study has some limitations. Firstly, the sample includes few women: less than 20% of our study participants were women. However, in terms of numbers, 113 women were followed in this study, which is a considerable figure, and moreover, their results are similar to those of men. Secondly, the cardiovascular comorbidities were not broken down or specified. Despite using a widely validated comorbidity index in this study, no differences were found between concomitant cardiovascular (CV) diseases (or the different types of CV disease), cancer, or other comorbidities. We do not know the burden of CV comorbidity among the subjects who had significant dyspnea. It has been reported that CV comorbidity can be as high as 20% in subjects with significant dyspnea,24 however, this does not seem to produce any bias in this study since we found no association between cardiovascular mortality and dyspnea.
In summary, this study demonstrates that the current classification of the severity of the GesEPOC guidelines has discriminative capacity in the prognosis of COPD patients. High-risk patients present a much higher mortality than low-risk patients, and within this high-risk group, mortality increases as more risk factors are added. Furthermore, exacerbations, as currently defined, are not related to mortality. Both dyspnea and FEV1% individually have the same prognostic power as the severity index proposed by GesEPOC.
Conflict of InterestsThe authors state that they have no conflict of interests.
Please cite this article as: Cabrera López C, Casanova Macario C, Marín Trigo JM, de-Torres JP, Torres RS, González JM. Validación pronóstica según los criterios de la GesEPOC 2017. Arch Bronconeumol. 2019;55:409–413.