Chronic obstructive pulmonary disease (COPD) and cardiovascular disease (CVD) are considered leading causes of death worldwide.1,2 Few studies have been carried out in patients with coronary artery disease in which standardized pulmonary functional tests were performed, but have often found a high prevalence of COPD and most patients were newly diagnosed.3–6
The main objective of our study is to determine the prevalence of airflow limitation (AL) in patients with ACS and, in addition, we aim to investigate which other clinical or sociodemographic factors, apart from those already known, may be predictors of undiagnosed AL compatible with COPD in such patients.
All consecutive patients with a diagnosis of ACS, hospitalized at the Arquitecto Marcide Hospital of Ferrol from January 1, 2019 to November 17, 2021, were evaluated for inclusion in the study. At least one month after discharge (median 49 days) a post-bronchodilator spirometry was performed, according to international guidelines,1 and reviewed by an experienced pulmonologist.
Forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC) were assessed with a spirometer (MasterScreen Body, Jaeger, Würzburg, Germany) and according to current international standards.7 AL compatible with a diagnosis of COPD was defined as a post-bronchodilator FEV1/FVC<0.70.8 Restricted spirometry was defined as pre-bronchodilator FEV1/FVC≥0.70 and FVC<80% of predicted,9 whereas combined obstructive-restricted spirometry was defined as post-bronchodilator FEV1/FVC<0.70 in combination with pre-bronchodilator FEV1/FVC≥0.70 and FVC<80% of predicted. Any other acceptable spirometry results were classified as normal lung function.
Patient's demographics, medical and treatment history were recorded. Data collected included the characteristic of the percutaneous coronary intervention due to SCA, smoking history, previous diagnosis of COPD, and risk factors for coronary artery disease. Patients with hemoglobin less than 12g/dl for women or 13g/dl for men were deemed to be anemic.
The research protocol was reviewed and approved by the Clinical Research Ethics Committee from Galicia, Spain (CEIC 2018/508). The study was conducted in accordance with the ethical principles of the Declaration of Helsinki.
The association between two continuous variables was tested using the Pearson correlation coefficient, and the association between two categorical or binary variables was assessed using the chi-square or Fisher exact test. Student's t-test or one-way analysis of variance (or Wilcoxon rank sum or Kruskal–Wallis tests, respectively, if normality of observations distribution could not be assumed) were used to assess the association between a categorical or binary variable and a continuous variable. The association between variables considered for abnormal lung function versus normal lung function was assessed by logistic regression models. Variables with a probability value less than 0.1 at univariate analysis and several potential confounding factors were then entered into a multivariate analysis to identify the independent predictors. Multivariate logistic regression analysis with backward elimination was conducted (or elimination threshold P>0.1) to determine the predictors of AL versus lung normal function. Variables such as age, gender, BMI, tobacco status, tobacco consumption, respiratory symptoms, comorbidities, hs-CRP, and NT-proBNP (>500pg/ml vs. ≤500pg/ml) were tested.
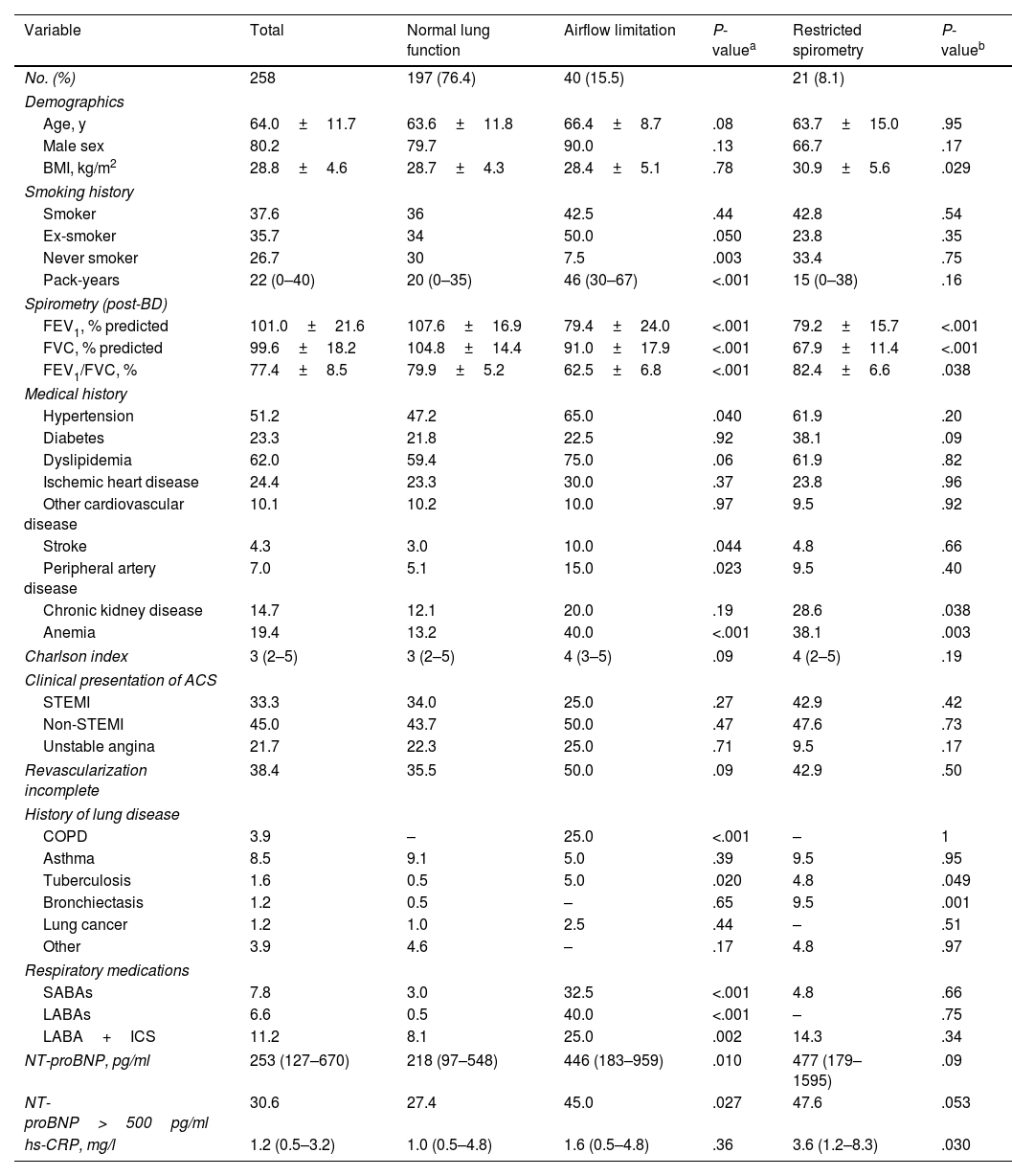
A total of 258 patients with acute myocardial infarction were included in this study. Clinical characteristics of patients according to lung function classification are shown in Table 1. Overall, 80.2% participants were men, the mean age was 64 years, and 37.6% were current smokers. A total of 17.1% reported having respiratory disease, including 3.9% who reported COPD. 33.3% of ACS patients presented with STEMI and 66.7% with non-STEMI. On average, patients with AL were slightly older (P=0.08), more often ex-smokers (P=0.05), and had more pack-years (P<0.001). In addition, there are significantly more cases of patients with hypertension (P=0.04), stroke (P=0.044), peripheral arterial disease (P=0.023), and anemia (P<0.0001).
Characteristics of participants with acute coronary syndrome stratified by lung function.
| Variable | Total | Normal lung function | Airflow limitation | P-valuea | Restricted spirometry | P-valueb |
|---|---|---|---|---|---|---|
| No. (%) | 258 | 197 (76.4) | 40 (15.5) | 21 (8.1) | ||
| Demographics | ||||||
| Age, y | 64.0±11.7 | 63.6±11.8 | 66.4±8.7 | .08 | 63.7±15.0 | .95 |
| Male sex | 80.2 | 79.7 | 90.0 | .13 | 66.7 | .17 |
| BMI, kg/m2 | 28.8±4.6 | 28.7±4.3 | 28.4±5.1 | .78 | 30.9±5.6 | .029 |
| Smoking history | ||||||
| Smoker | 37.6 | 36 | 42.5 | .44 | 42.8 | .54 |
| Ex-smoker | 35.7 | 34 | 50.0 | .050 | 23.8 | .35 |
| Never smoker | 26.7 | 30 | 7.5 | .003 | 33.4 | .75 |
| Pack-years | 22 (0–40) | 20 (0–35) | 46 (30–67) | <.001 | 15 (0–38) | .16 |
| Spirometry (post-BD) | ||||||
| FEV1, % predicted | 101.0±21.6 | 107.6±16.9 | 79.4±24.0 | <.001 | 79.2±15.7 | <.001 |
| FVC, % predicted | 99.6±18.2 | 104.8±14.4 | 91.0±17.9 | <.001 | 67.9±11.4 | <.001 |
| FEV1/FVC, % | 77.4±8.5 | 79.9±5.2 | 62.5±6.8 | <.001 | 82.4±6.6 | .038 |
| Medical history | ||||||
| Hypertension | 51.2 | 47.2 | 65.0 | .040 | 61.9 | .20 |
| Diabetes | 23.3 | 21.8 | 22.5 | .92 | 38.1 | .09 |
| Dyslipidemia | 62.0 | 59.4 | 75.0 | .06 | 61.9 | .82 |
| Ischemic heart disease | 24.4 | 23.3 | 30.0 | .37 | 23.8 | .96 |
| Other cardiovascular disease | 10.1 | 10.2 | 10.0 | .97 | 9.5 | .92 |
| Stroke | 4.3 | 3.0 | 10.0 | .044 | 4.8 | .66 |
| Peripheral artery disease | 7.0 | 5.1 | 15.0 | .023 | 9.5 | .40 |
| Chronic kidney disease | 14.7 | 12.1 | 20.0 | .19 | 28.6 | .038 |
| Anemia | 19.4 | 13.2 | 40.0 | <.001 | 38.1 | .003 |
| Charlson index | 3 (2–5) | 3 (2–5) | 4 (3–5) | .09 | 4 (2–5) | .19 |
| Clinical presentation of ACS | ||||||
| STEMI | 33.3 | 34.0 | 25.0 | .27 | 42.9 | .42 |
| Non-STEMI | 45.0 | 43.7 | 50.0 | .47 | 47.6 | .73 |
| Unstable angina | 21.7 | 22.3 | 25.0 | .71 | 9.5 | .17 |
| Revascularization incomplete | 38.4 | 35.5 | 50.0 | .09 | 42.9 | .50 |
| History of lung disease | ||||||
| COPD | 3.9 | – | 25.0 | <.001 | – | 1 |
| Asthma | 8.5 | 9.1 | 5.0 | .39 | 9.5 | .95 |
| Tuberculosis | 1.6 | 0.5 | 5.0 | .020 | 4.8 | .049 |
| Bronchiectasis | 1.2 | 0.5 | – | .65 | 9.5 | .001 |
| Lung cancer | 1.2 | 1.0 | 2.5 | .44 | – | .51 |
| Other | 3.9 | 4.6 | – | .17 | 4.8 | .97 |
| Respiratory medications | ||||||
| SABAs | 7.8 | 3.0 | 32.5 | <.001 | 4.8 | .66 |
| LABAs | 6.6 | 0.5 | 40.0 | <.001 | – | .75 |
| LABA+ICS | 11.2 | 8.1 | 25.0 | .002 | 14.3 | .34 |
| NT-proBNP, pg/ml | 253 (127–670) | 218 (97–548) | 446 (183–959) | .010 | 477 (179–1595) | .09 |
| NT-proBNP>500pg/ml | 30.6 | 27.4 | 45.0 | .027 | 47.6 | .053 |
| hs-CRP, mg/l | 1.2 (0.5–3.2) | 1.0 (0.5–4.8) | 1.6 (0.5–4.8) | .36 | 3.6 (1.2–8.3) | .030 |
Data are presented as percentage, mean±SD, or median (interquartile range), unless otherwise indicated. ACS=acute coronary syndrome; hs-CRP=high-sensitivity C-reactive protein; ICS=inhaled corticosteroids; LABA=long acting β2-adrenoceptor agonists; NSTEMI=non-ST-elevation myocardial infarction; NT-proBNP=N-terminal pro B-type natriuretic peptide; post-BD=post-bronchodilator; SABA=short-acting β2-adrenoceptor agonist; and STEMI=ST-elevation myocardial infarction.
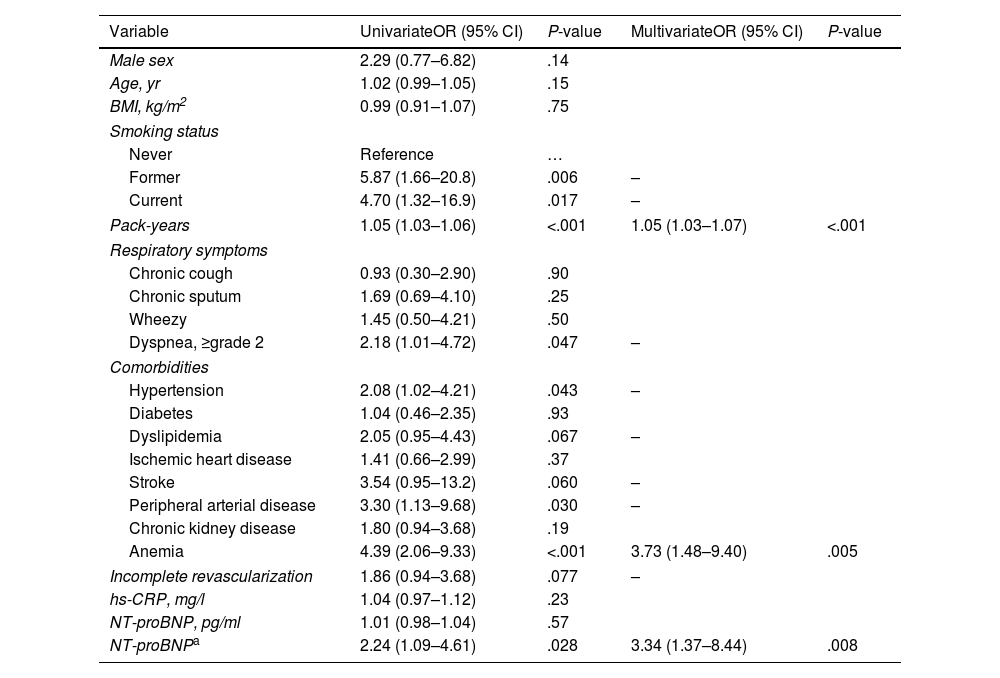
In univariate analysis, AL was associated with variables such as current or ex-smokers (vs never smoking), greater smoking history, grade of dyspnea, hypertension, peripherical artery disease, anemia, and patients with NT-proBNP>500pg/ml (vs NT-proBNP≤500pg/ml).
Using a multivariate stepwise logistic regression model, we identified three variables as being independently associated with the presence of AL versus normal lung function: pack-years, anemia, and NT-proBNP>500pg/ml (Table 2).
Univariate and Multivariate Associations of Risk Factors for Airflow Limitation Versus Normal Lung Function in Patients with Acute Coronary Syndrome.
| Variable | UnivariateOR (95% CI) | P-value | MultivariateOR (95% CI) | P-value |
|---|---|---|---|---|
| Male sex | 2.29 (0.77–6.82) | .14 | ||
| Age, yr | 1.02 (0.99–1.05) | .15 | ||
| BMI, kg/m2 | 0.99 (0.91–1.07) | .75 | ||
| Smoking status | ||||
| Never | Reference | … | ||
| Former | 5.87 (1.66–20.8) | .006 | – | |
| Current | 4.70 (1.32–16.9) | .017 | – | |
| Pack-years | 1.05 (1.03–1.06) | <.001 | 1.05 (1.03–1.07) | <.001 |
| Respiratory symptoms | ||||
| Chronic cough | 0.93 (0.30–2.90) | .90 | ||
| Chronic sputum | 1.69 (0.69–4.10) | .25 | ||
| Wheezy | 1.45 (0.50–4.21) | .50 | ||
| Dyspnea, ≥grade 2 | 2.18 (1.01–4.72) | .047 | – | |
| Comorbidities | ||||
| Hypertension | 2.08 (1.02–4.21) | .043 | – | |
| Diabetes | 1.04 (0.46–2.35) | .93 | ||
| Dyslipidemia | 2.05 (0.95–4.43) | .067 | – | |
| Ischemic heart disease | 1.41 (0.66–2.99) | .37 | ||
| Stroke | 3.54 (0.95–13.2) | .060 | – | |
| Peripheral arterial disease | 3.30 (1.13–9.68) | .030 | – | |
| Chronic kidney disease | 1.80 (0.94–3.68) | .19 | ||
| Anemia | 4.39 (2.06–9.33) | <.001 | 3.73 (1.48–9.40) | .005 |
| Incomplete revascularization | 1.86 (0.94–3.68) | .077 | – | |
| hs-CRP, mg/l | 1.04 (0.97–1.12) | .23 | ||
| NT-proBNP, pg/ml | 1.01 (0.98–1.04) | .57 | ||
| NT-proBNPa | 2.24 (1.09–4.61) | .028 | 3.34 (1.37–8.44) | .008 |
BMI=body mass index; hs-CRP=high-sensitivity C-reactive protein; and NT-proBNP=N-terminal pro B-type natriuretic peptide; (–)=not included in the model, not statistically significant in multivariate analyses.
In the population of ACS patients studied, the prevalence of AL was 15.5%; however, if only patients with smoking history ≥10 pack-years are considered, the total prevalence raised up to 21.5% (eTable 1).
In patients without anemia (n=208), the prevalence of AL was 11.5% versus 32% in those with anemia (n=50) (P<0.001) (eTable 1). A smaller increase in cases of AL was observed among patients with NT-proBNP levels≤500pg/ml versus patients with levels>500pg/ml (12.0 vs. 21.5%, P=0.052).
Among the 258 patients with ACS, 40 had CODP and the percentage of undiagnosed was 75%. A relevant finding of our study is that among undiagnosed patients 36.7% had anemia, but its value increases up to 56.7% if patients without anemia having a history of smoking and NT-proBNP>500pg/ml are included.
Very few studies were performed in patients with ACS who had undergone standardized spitometry.3–6 In these studies the prevalence of AL range from 11 to 29%, which is similar to 15.5% we have found in our ACS patients, but it is higher than 10.6% found by Mooe,5 although up to 35% of his patients did not smoke against to 26.7% did not in our study.
In Campo's study4 the prevalence of AL was higher (28.5%); but patients who never smoked were excluded. In a large study including patients with stablished ischemic heart disease,10 30.5% of patients had COPD by spirometric criteria; but all participants were (ex-)smokers and had stablished ischemic heart disease and no ACS. In our study, if only patients with smoking history greater than 10 pack-years are taken into account the total prevalence of AL increases from 15.5% to 21.5%.
We also found a 75% of patients with undiagnosed AL, which is generally and consistently indicated by data from previous studies.11-13
The main independent risk factors for AL that we were able to identify in our patients were smoking history, anemia, and circulating NT-proBNP levels. The smoking history was largely confirmed in previous studies10-12,14; however, as far as we known, anemia and NT-proBNP levels have never been identified as independent risk factors, neither in the general population nor in patients with different cardiovascular diseases. The direct association between risk for AL and levels of NT-proBNP>500pg/ml in our patients is in line with the high prevalence of COPD in patients with heart failure.15
Many studies have previously described an association between anemia and COPD morbidity.16–19 In our study, the prevalence of anemia was 19.4%, and the probability of AL in patients with ACS having anemia is 3.7 times higher than patients without anemia, and it was independent of age, sex, smoking history, respiratory symptoms, comorbidities, and circulating levels of NT-proBNP or hs-CR.
Adequate detection of anemia in these patients and assessment of different treatment options is supported by our results. Indeed, each increase in hemoglobin levels by 1g/dl is associated to a 36% reduction in the prevalence of AL.
In conclusion, we observed a significant higher prevalence of chronic AL in patients with ACS than in general population and the majority of patients were undiagnosed. We also observed that, in addition to tobacco use, anemia and elevated NT-proBNP levels are important risk factors for the development of AL. We conclude that performing spirometry in those patients with ACS having these risk factors could identify up to 60% of new cases of AL. However, further studies are needed to assess whether the impact of these risk factors is generalizable to other populations.
Authors’ contributionsSR-SA conceived the study, developed the statistical analysis with SR-S assistance. Both drafted the manuscript and are the guarantors of this work, and as such, had full access to the data in the study and take responsibility for the integrity and the accuracy of the data. SR-SA, CDR, RMR, INC and ECF undertook patient visits. All authors contributed to research data and critically reviewed the manuscript for important intellectual content. SR-SA, FC and SR-S edited the final version of the manuscript and made the artwork. All Authors approved the final version of the manuscript.
Data sharingData are available upon reasonable request from the corresponding Author. All data relevant to the study are included in the article or uploaded as supplementary information.
FundingsThis research received no specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflict of interestSR-SA declares honoraria for presentations in educational events from AstraZeneca (2021) and GSK (2021). This research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in study design, data collection and analysis, preparation of the manuscript, or the submission process.
We gratefully to all participants in this research.