The impact of preoperative nutritional status on survival in lung cancer (LC) patients with underlying chronic obstructive pulmonary disease (COPD) is still unclear. We hypothesized that presurgical nutritional assessment may differentially predict mortality in patients with resectable LC with moderate COPD and relatively well-preserved nutritional status.
MethodsNutritional assessment [body mass index (BMI), blood parameters including albumin and protein levels, and body weight loss], and other clinical parameters [cigarette smoking (CS) history, LC staging and histological subtypes, COPD severity, lung function, and adjuvant therapy] were evaluated in 125 patients from the LC Mar Prospective Cohort: 87 LC-COPD patients and 38 LC patients without COPD before thoracotomy. Ten-year overall survival (OS) was analyzed in all patients.
ResultsPrior to thoracotomy, in LC-COPD patients compared to LC, BMI and albumin declined relatively, low levels of the parameters BMI, albumin, and total proteins were associated with poorer 10-year survival, especially in the LC-COPD. CS burden also correlated with impaired survival. COPD per se worsened the prognosis in LC patients.
ConclusionsIn the present cohort of LC patients with resectable tumors and relatively well-preserved nutritional status, the parameters BMI and blood albumin and protein levels measured prior to thoracotomy predicted OS, especially in those with COPD. These are clinically relevant findings, since values of those nutritional parameters were within the normal ranges in the majority of the analyzed patients. A thorough nutritional preoperative assessment should be included in the study of patients with resectable LC, particularly in those with chronic airway obstruction.
El impacto del estado nutricional preoperatorio en la supervivencia en pacientes con cáncer de pulmón (CP) y enfermedad pulmonar obstructiva crónica (EPOC) subyacente aún no está claro. Planteamos la hipótesis de que la evaluación nutricional prequirúrgica puede predecir diferencialmente la mortalidad en pacientes con CP resecable y EPOC moderada, y un estado nutricional relativamente bien conservado.
MétodosSe evaluaron el estado nutricional (índice de masa corporal [IMC], parámetros sanguíneos que incluyeron los niveles de albúmina y proteínas y pérdida de peso corporal) y otros parámetros clínicos (antecedentes de tabaquismo, estadificación del CP y el subtipo histológico, gravedad de la EPOC, función pulmonar y terapia adyuvante) en 125 pacientes de la cohorte prospectiva de CP del Hospital del Mar: 87 pacientes con EPOC-CP y 38 pacientes con CP sin EPOC antes de la toracotomía. Se analizó la supervivencia global (SG) a 10 años en todos los pacientes.
ResultadosAntes de la toracotomía, en los pacientes con EPOC-CP, el IMC y la albúmina disminuyeron en comparación con los del grupo de CP; los niveles bajos de los parámetros IMC, albúmina y proteínas totales se asociaron con menor supervivencia a 10 años, especialmente en los EPOC-CP. La carga tabáquica también se correlacionó con una disminución en la supervivencia. La EPOC empeoró per se el pronóstico en pacientes con CP.
ConclusionesEn la presente cohorte de pacientes con CP resecable y estado nutricional relativamente bien conservado, el IMC y los niveles de albúmina y proteínas en sangre medidos antes de la toracotomía predijeron la SG, especialmente en aquellos con EPOC. Estos son hallazgos clínicamente relevantes, ya que los valores de esos parámetros nutricionales estaban dentro de los rangos normales en la mayoría de los pacientes analizados. Se debe incluir una evaluación nutricional preoperatoria exhaustiva en el estudio de pacientes con CP resecable, particularmente en aquellos con obstrucción pulmonar crónica.
Lung cancer (LC) is a major cause of mortality among different cancer types worldwide.1–5 Several etiologic factors including underlying respiratory diseases have been reported in the literature.6–9 Chronic obstructive pulmonary disease (COPD) has been consistently demonstrated to predispose patients to cancer development.6–9 COPD is also a high prevalent condition worldwide and is associated with many different types of comorbidities.3,10,11 Skeletal muscle dysfunction and alterations in body composition and nutritional abnormalities are counted among the most clinically relevant ones as they impact disease prognosis and survival in COPD patients.12
In LC and COPD patients, it has been well-established that body weight loss and altered nutritional parameters as disease progresses have a negative impact in their survival. Recently, it has been demonstrated that in patients with resected LC, the level of body mass index (BMI) before the surgery predicted the overall survival (OS) of the patients that were followed up for several years.13 In other cancer types,14 a 5% reduction in BMI significantly increased the risk of mortality, especially in the elderly. In another recent investigation,15 baseline body weight loss and albumin had a negative impact on prognosis in patients with LC treated with immunotherapy. Moreover, other investigations have also reported that nutritional abnormalities predicted survival in LC patients with advanced stages,16 impaired clinical outcomes,17 and postoperative complications.18,19 Whether nutritional status may also predict OS in LC patients with underlying respiratory conditions, namely COPD and preserved body composition remain to be elucidated.
On this basis, we hypothesized that clinical parameters defining nutrition prior to surgery may differentially predict mortality in patients with resectable LC with moderate COPD and relatively well-preserved nutritional status. Accordingly, the following objectives were established. In a prospective cohort of LC patients with and without COPD the associations between preserved preoperative nutritional status (BMI, albumin, and total protein levels) and OS were analyzed. All patients were followed up to a period of ten years.
MethodsStudy design and ethicsThis is a prospective study designed following the World Medical Association guidelines (Seventh revision of the Declaration of Helsinki, Fortaleza, Brazil, 2013)20 for research on human beings and approved by the institutional Ethics Committee on Human Investigation (Hospital del Mar–IMIM, Barcelona, Spain). All patients invited to participate in the study signed the written informed consent.
Patients were prospectively recruited from the Lung Cancer Clinic of the Respiratory Medicine Department at Hospital del Mar (Barcelona, Spain). All the patients were part of the Lung Cancer Mar Cohort that started in 2008. For this observational study, 125 patients with LC were recruited. Patients were further subdivided post hoc into two groups on the basis of underlying COPD: (1) 87 patients with LC and COPD (LC-COPD group) and (2) 38 patients with LC without COPD (LC group). These patients were simultaneously participating in another investigation aimed to explore the associations between the immune microenvironment (B cells and tertiary lymphoid structures) in the lung tumor specimens and OS of the patients (submitted to another journal).
Study patientsLC diagnosis and staging were established by histological confirmation and classified according to currently available guidelines for the diagnosis and management of LC.21,22 TNM (tumor, node, and metastasis) staging was defined as stated in the 8th edition Lung Cancer Stage Classification.23 In all cases, pre-operative staging was performed using chest and upper abdomen Computed Tomography (CT) scan and Fluoro-deoxy-glucose positron emission tomography/computed tomography (PET) body-scan. When suspected mediastinal lymph-node involvement, a fiberoptic bronchoscopy with endo-bronchial ultra-sound (EBUS) and trans-tracheal biopsy of the suspected nodes were performed. In case of negative results, a surgical exploration of the mediastinum: cervical video-assisted mediastinal lymphadenectomy (VAMLA) and/or anterior mediastinotomy were performed, the latter depending on the location of the suspected nodes. Notwithstanding, in all surgical cases, intra-operative systematic hilar and mediastinal lymphadenectomy (at least, ipsilateral paratracheal, subcarinal, and ipsilateral pulmonary ligament) was performed as previously recommended.24,25 Standard clinical guidelines were used to establish the selection of patients and contraindications for thoracic surgery as previously described.26 Decisions on the best therapeutic approach were always made during the weekly meetings of the Multidisciplinary Lung Cancer Committee. Lung tumor resections were applied using classical thoracotomy for all the patients in this study.
In the current investigation, exclusion criteria for the participants were established as follows: small cell lung cancer (SCLC), severe malnutrition status, chronic cardiovascular disease, metabolic or clot system disorders, signs of severe inflammation and/or bronchial infection (bronchoscopy), current or recent invasive mechanical ventilation, or long-term oxygen therapy. The presence/absence of these diseases was confirmed using standard clinical tests: exercise capacity electrocardiogram, clinical examination, blood tests, bronchoscopy and echocardiography.
Clinical assessmentIn all patients, lung function parameters were assessed following standard procedures. Diagnosis and severity of patients with COPD were determined according to current guidelines.10 Preoperative nutritional evaluation included the assessment of body mass index (BMI) and nutritional blood parameters from all patients. All the parameters were quantified prior to thoracotomy for their lung neoplasm. Moreover, body weight loss over the previous year to study entry was also quantified in all the study patients. Body weight loss during follow-up was not quantified routinely in patients of this study.
Statistical analysesThe normality of the study variables was examined using the Shapiro–Wilk test. Accepting an alpha risk of 0.05 and a beta risk of 0.2 in a two-sided test, 33 subjects were necessary in the LC group and 66 in the LC-COPD group to identify a statistically significant difference greater than or equal to 3 units in mean of BMI variable. The common standard deviation was assumed as 5. The number of patients in each group was calculated using GRANMO (IMIM, Barcelona). Moreover, taking each variable categorized into two groups, estimated power for two-sample comparisons of survivor functions Log-rank test was applied using the Freedman method. Accepting an alpha risk of 0.05 in a two-sided test with 87 and 38 patients in each group (post hoc subdivision), the statistical power was 85% (albumin), BMI (76%), and 100% (FEV1).
For the descriptive analysis of clinical parameters, qualitative variables were described as frequencies (number and percentage) and quantitative variables as mean and standard deviation. Differences in clinical parameters between LC and LC-COPD were assessed using Student's T-test or Mann–Whitney U tests for parametric and non-parametric variables, respectively. Chi-square test was used to assess differences between the two groups for the categorical variables.
OS was defined as the time from the date of diagnosis of LC to the date of death from this disease or the last follow-up which was completed in December 2018. The median follow-up duration was 37.7 months (P25=20.4 months, P75=60.5 months). Patients were followed for a minimum of one year and up to maximum period of 10 years. Patients who did not died of lung cancer were excluded in the investigation. Two patients who were lost during follow-up were not included in the OS analyses. Threshold analysis was carried out for each continuous variable to determine the best cut-off point as predictor of OS, which was the endpoint in the study. The cut-off point was defined using the web-based software Cutoff Finder,27 which has also been previously used in other studies.28,29 For each variable, we identified the threshold level at which a log-rank test allowed segregation of patients into groups with better and worse survival. Kaplan–Meier survival curves were performed for each dichotomized variable (below versus above cutoff values, described as Lo and Hi) and log-rank test p-value was estimated.
Univariate and multivariate Cox regression models were used to study OS among all LC patients. Those variables with p-value smaller than 0.1 were entered into the multivariate analyses. The proportional hazard assumption, checked by examining Schoenfeld residuals (for overall model and variable by variable), was not violated. Statistical significance was established at p≤0.05. All statistical analyses were carried out using the software Stata/MP 15 (StataCorp LLC, Texas, USA).
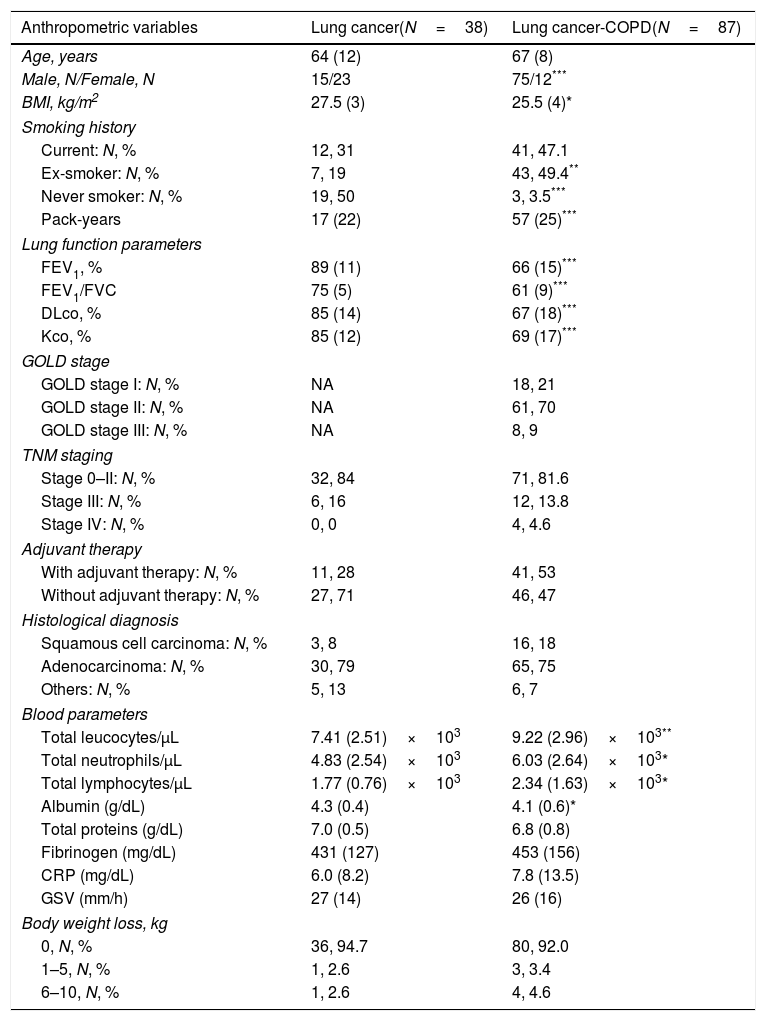
ResultsClinical characteristicsNo significant differences were seen in age between the two study groups (Table 1). In this prospective study, the number of LC-COPD patients was greater than patients in the LC group (Table 1). BMI, although within normal ranges, was significantly lower in LC-COPD than in LC patients (Table 1). The number of male patients among the LC-COPD group was significantly higher than in LC group (Table 1). Smoking history including packs-year was significantly worse in the LC-COPD patients than in LC group (Table 1). In LC-COPD patients, the lung functional parameters FEV1, FEV1/FVC, DLCO and KCO were significantly reduced compared to LC patients (Table 1). COPD patients were mostly in GOLD I and II stages (91%). TNM staging or histological subtypes did not significantly differ between the two groups. The proportions of patients that received adjuvant therapy did not significantly differ between the two study groups (Table 1).
Clinical and functional characteristics of the study patients.
| Anthropometric variables | Lung cancer(N=38) | Lung cancer-COPD(N=87) |
|---|---|---|
| Age, years | 64 (12) | 67 (8) |
| Male, N/Female, N | 15/23 | 75/12*** |
| BMI, kg/m2 | 27.5 (3) | 25.5 (4)* |
| Smoking history | ||
| Current: N, % | 12, 31 | 41, 47.1 |
| Ex-smoker: N, % | 7, 19 | 43, 49.4** |
| Never smoker: N, % | 19, 50 | 3, 3.5*** |
| Pack-years | 17 (22) | 57 (25)*** |
| Lung function parameters | ||
| FEV1, % | 89 (11) | 66 (15)*** |
| FEV1/FVC | 75 (5) | 61 (9)*** |
| DLco, % | 85 (14) | 67 (18)*** |
| Kco, % | 85 (12) | 69 (17)*** |
| GOLD stage | ||
| GOLD stage I: N, % | NA | 18, 21 |
| GOLD stage II: N, % | NA | 61, 70 |
| GOLD stage III: N, % | NA | 8, 9 |
| TNM staging | ||
| Stage 0–II: N, % | 32, 84 | 71, 81.6 |
| Stage III: N, % | 6, 16 | 12, 13.8 |
| Stage IV: N, % | 0, 0 | 4, 4.6 |
| Adjuvant therapy | ||
| With adjuvant therapy: N, % | 11, 28 | 41, 53 |
| Without adjuvant therapy: N, % | 27, 71 | 46, 47 |
| Histological diagnosis | ||
| Squamous cell carcinoma: N, % | 3, 8 | 16, 18 |
| Adenocarcinoma: N, % | 30, 79 | 65, 75 |
| Others: N, % | 5, 13 | 6, 7 |
| Blood parameters | ||
| Total leucocytes/μL | 7.41 (2.51)×103 | 9.22 (2.96)×103** |
| Total neutrophils/μL | 4.83 (2.54)×103 | 6.03 (2.64)×103* |
| Total lymphocytes/μL | 1.77 (0.76)×103 | 2.34 (1.63)×103* |
| Albumin (g/dL) | 4.3 (0.4) | 4.1 (0.6)* |
| Total proteins (g/dL) | 7.0 (0.5) | 6.8 (0.8) |
| Fibrinogen (mg/dL) | 431 (127) | 453 (156) |
| CRP (mg/dL) | 6.0 (8.2) | 7.8 (13.5) |
| GSV (mm/h) | 27 (14) | 26 (16) |
| Body weight loss, kg | ||
| 0, N, % | 36, 94.7 | 80, 92.0 |
| 1–5, N, % | 1, 2.6 | 3, 3.4 |
| 6–10, N, % | 1, 2.6 | 4, 4.6 |
Continuous variables are presented as mean and standard deviation while categorical variables are presented as the number of patients in each group and the percentage in the study group with respect to the total population. Definition of abbreviations: N, number; kg, kilograms; m, meters; BMI, body mass index; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; DLCO, carbon monoxide transfer; KCO, Krogh transfer factor; GOLD, Global initiative for chronic Obstructive Lung Disease; NA, not applicable; TNM, tumor, nodes, metastasis; CRP, C-reactive protein; GSV, globular sedimentation velocity; L, liter. Statistical analyses and significance.
Total leukocyte, neutrophil and lymphocyte counts were significantly greater in LC-COPD patients (Table 1). However, albumin levels were significantly reduced in LC-COPD patients (Table 1). No significant differences were shown in levels of total proteins, fibrinogen, C-reactive protein (CRP), globular sedimentation velocity (GSV) between LC-COPD and LC patients (Table 1). Body weight loss did not significantly differ between the study groups (Table 1). In LC-COPD group of patients, only four patients (2 current smokers and 2 ex-smokers) experienced the greatest weight loss range (6–10kg) within the previous 12 months before study entry (Table 1). In LC group, only one smoker patient exhibited a great range of weight loss (Table 1).
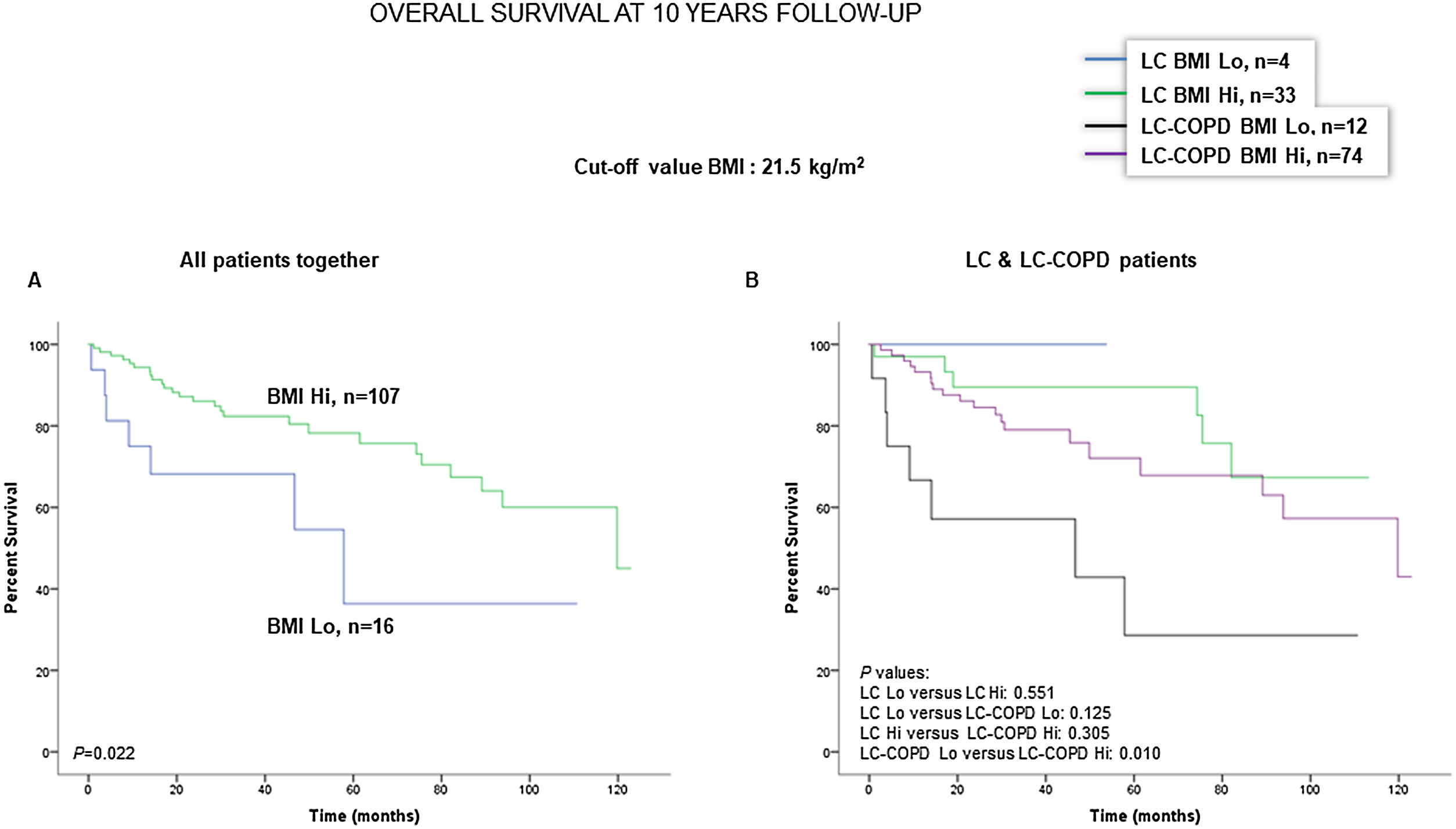
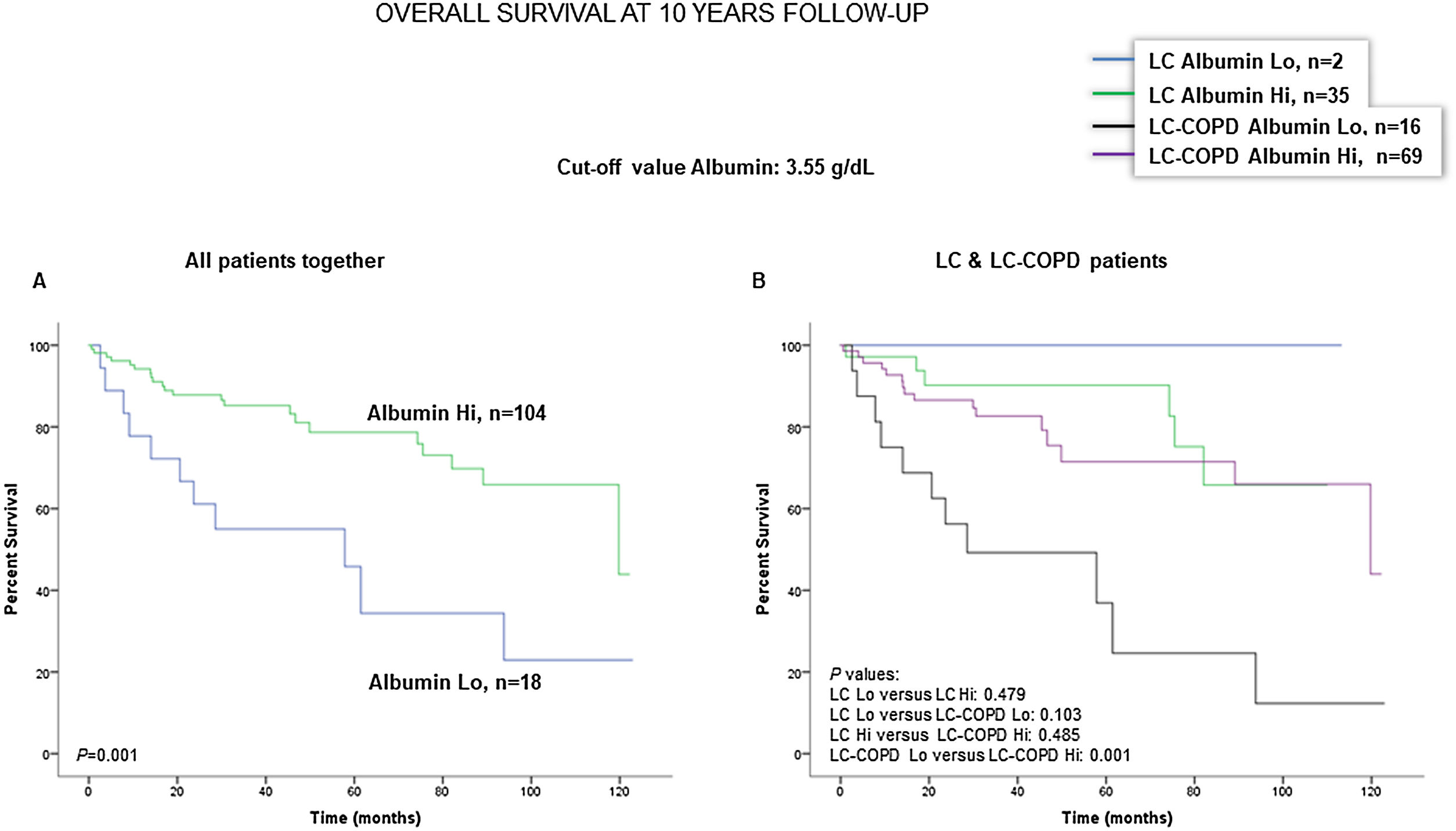
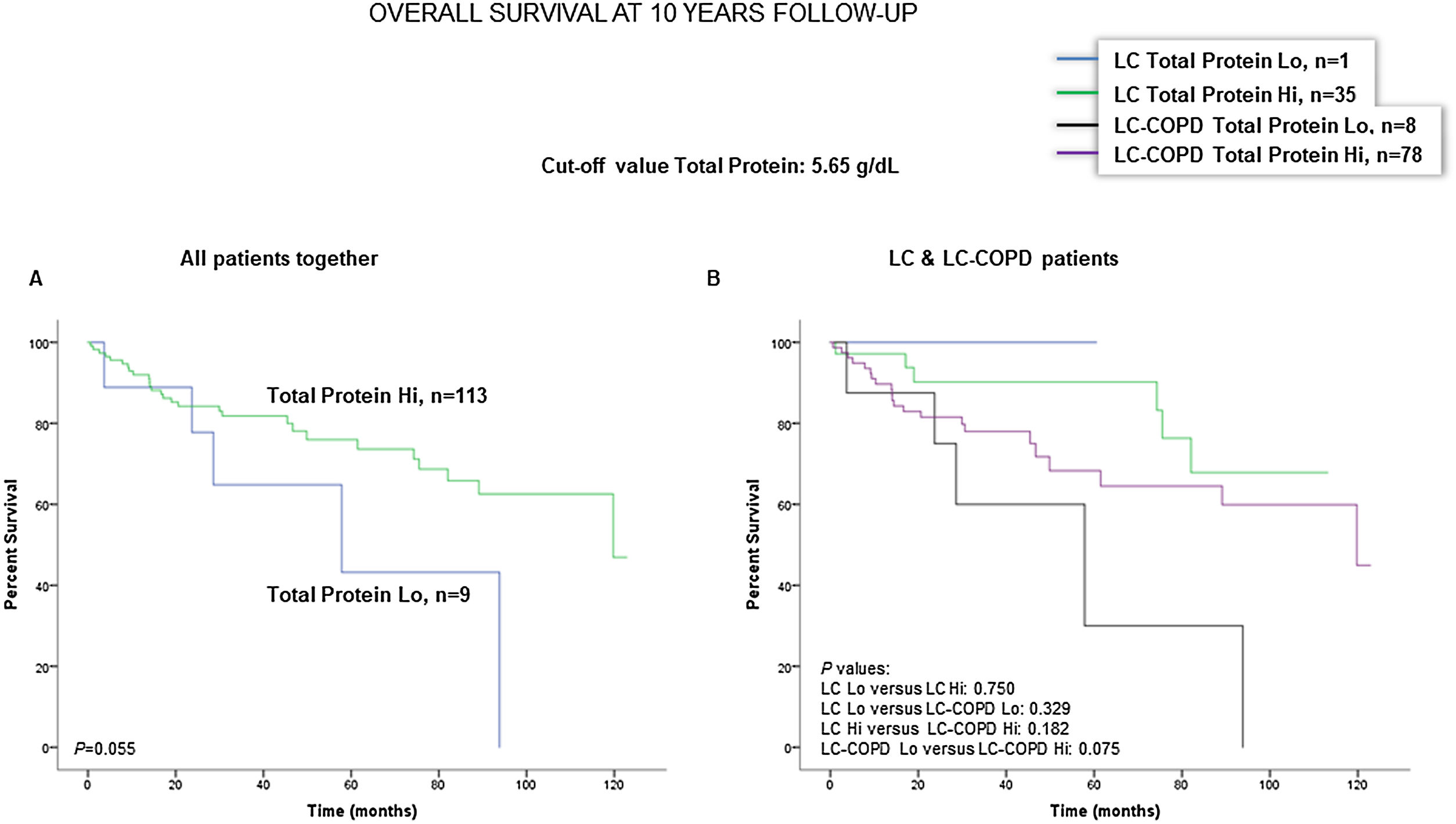
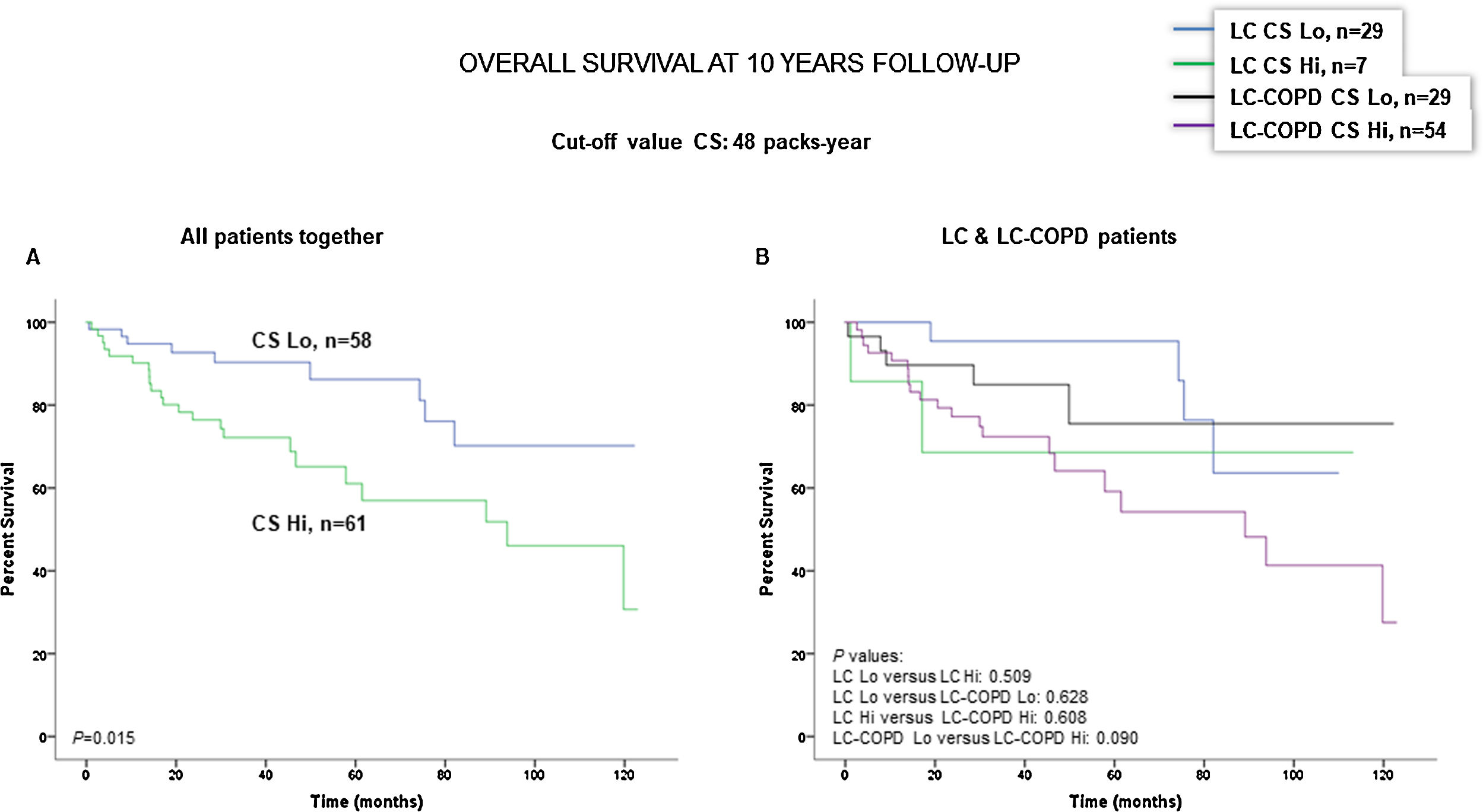
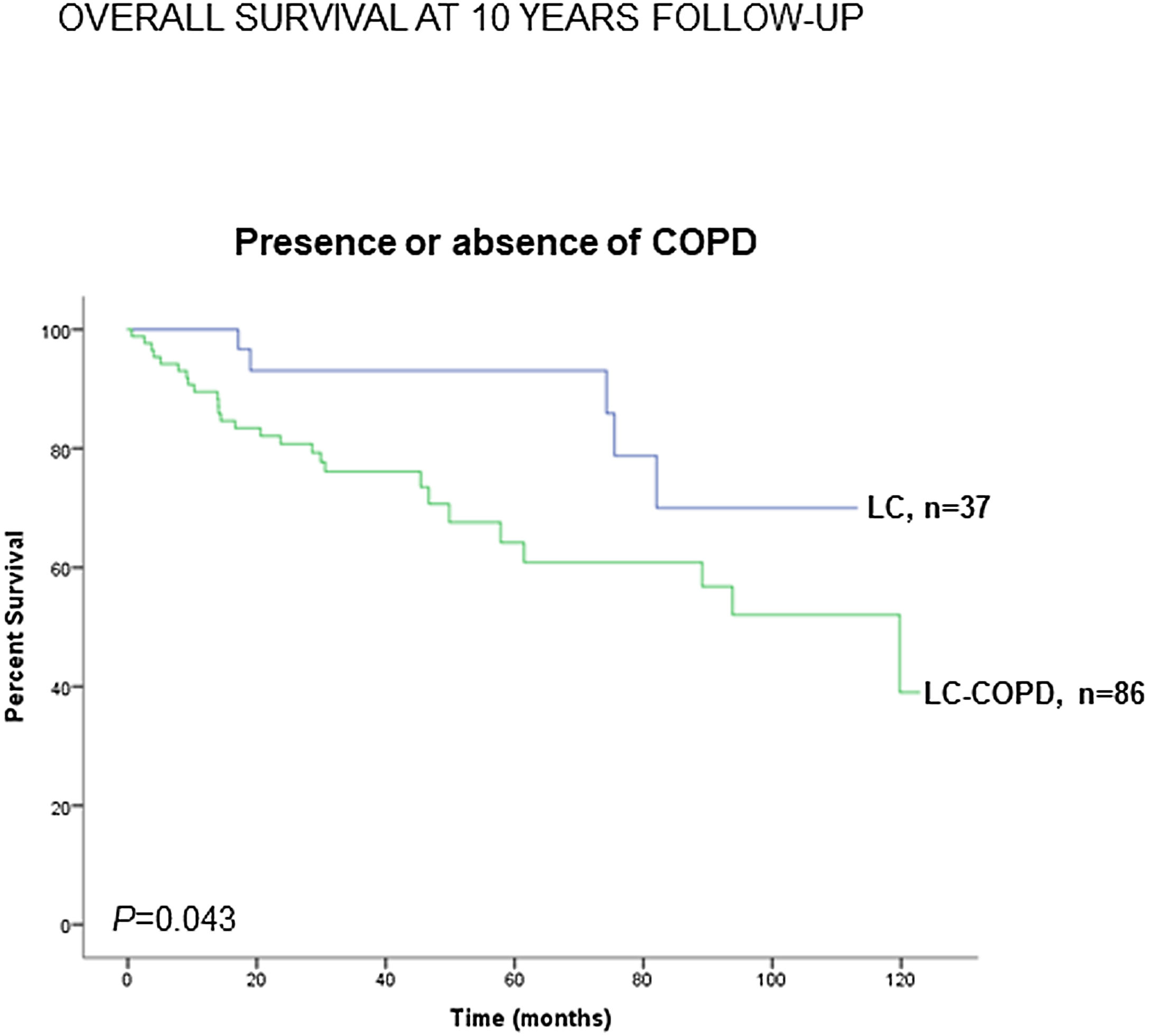
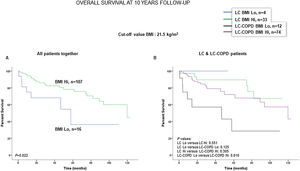
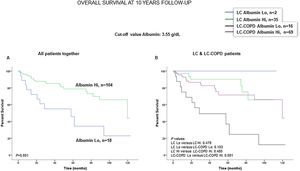
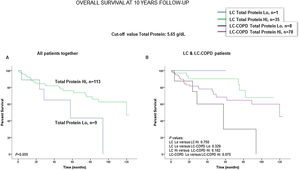
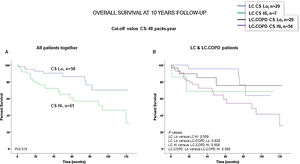
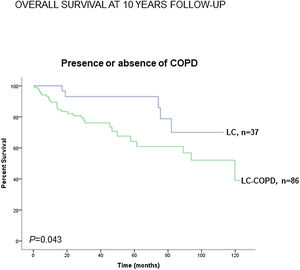
Preoperative nutritional variables, cigarette smoking, and OS in LC and LC-COPD patientsWhen all patients were analyzed together, a lower level of BMI (cut-off: 21.5kg/m2) was associated with a poorer 10-year survival (Fig. 1A). When patients were subdivided according to the presence of COPD, a significant worse ten-year survival was observed in LC-COPD patients with a lower degree of BMI (Fig. 1B). Similar results were observed when only ex-smokers and never-smokers were analyzed independently (Fig. S1A and S1B). As to the levels of albumin, when all patients were analyzed together, a lower level of albumin (cut-off: 3.55g/dL) was associated with a poorer 10-year survival (Fig. 2A). When patients were subdivided according to the presence of COPD, a significant worse survival was observed in LC-COPD patients with lower levels of albumin (Fig. 2B). Similar findings were also obtained when non-smokers (never smokers and ex-smokers) were analyzed separately (Fig. S2A and S2B). The patients’ 10-year survival was almost significantly reduced in patients with lower levels of total proteins (cut-off: 5.65/dL) when patients were analyzed altogether (p=0.055) and in the group of LC-COPD (p=0.075, Fig. 3A and B, respectively). Non-significant differences were observed in OS when non-smokers (never-smokers and ex-smokers) were analyzed independently (Fig. S3A and S3B). Cigarette smoking was also analyzed in the study. When all patients were analyzed together higher levels of cigarette smoking burden (cut-off: 48 packs-year) were associated with a poorer 10-year survival (Fig. 4A). Moreover, when patients were subdivided according to the presence of COPD, an almost significant worse survival (p=0.090) was observed in LC-COPD patients with a greater cigarette smoking burden (Fig. 4B). Interestingly, no significant differences were seen in OS when non-smoker patients were analyzed separately (Fig. S4A and S4B). In the study cohort, the presence of underlying COPD per se was also significantly associated with a lower 10-year survival as shown in Fig. 5. Consistently, no significant differences were seen when non-smokers were analyzed independently (Fig. S5).
(A) Kaplan–Meier survival curves for OS in all patients based on the cut-off value of the BMI (above and below the cut-off value: 21.5kg/m2). (B) Kaplan–Meier survival curves for OS in LC patients with and without COPD based on the cut-off value of the BMI (above and below the cut-off value: 21.5kg/m2). This information was not available in two patients. Definition of abbreviations: BMI, body mass index; LC, lung cancer; COPD, chronic obstructive pulmonary disease; Hi, high level (above cut-off value); Lo, low level (below cut-off value).
(A) Kaplan–Meier survival curves for OS in all patients based on the cut-off value of the albumin level (above and below the cut-off value: 3.55g/dL) in blood. (B) Kaplan–Meier survival curves for OS in LC patients with and without COPD based on the cut-off value of the albumin level (above and below the cut-off value: 3.55g/dL) in blood. This information was not available in three patients. Definition of abbreviations: LC, lung cancer; COPD, chronic obstructive pulmonary disease; Hi, high level (above cut-off value); Lo, low level (below cut-off value).
(A) Kaplan–Meier survival curves for OS in all patients based on the cut-off value of the total protein level (above and below the cut-off value: 5.65g/dL) in blood. (B) Kaplan–Meier survival curves for OS in LC patients with and without COPD based on the cut-off value of protein levels (above and below the cut-off value: 5.65g/dL) in blood. This information was not available in three patients. Definition of abbreviations: LC, lung cancer; COPD, chronic obstructive pulmonary disease; Hi, high level (above cut-off value); Lo, low level (below cut-off value).
(A) Kaplan–Meier survival curves for OS in all patients based on the cut-off value of cigarette smoking burden (above and below the cut-off value: 48 packs-year). (B) Kaplan–Meier survival curves for OS in LC patients with and without COPD based on the cut-off value of cigarette smoking burden (above and below the cut-off value: 48 packs-year). This information was not available in six patients. Definition of abbreviations: LC, lung cancer; COPD, chronic obstructive pulmonary disease; CS, cigarette smoking; Hi, high level (above cut-off value); Lo, low level (below cut-off value).
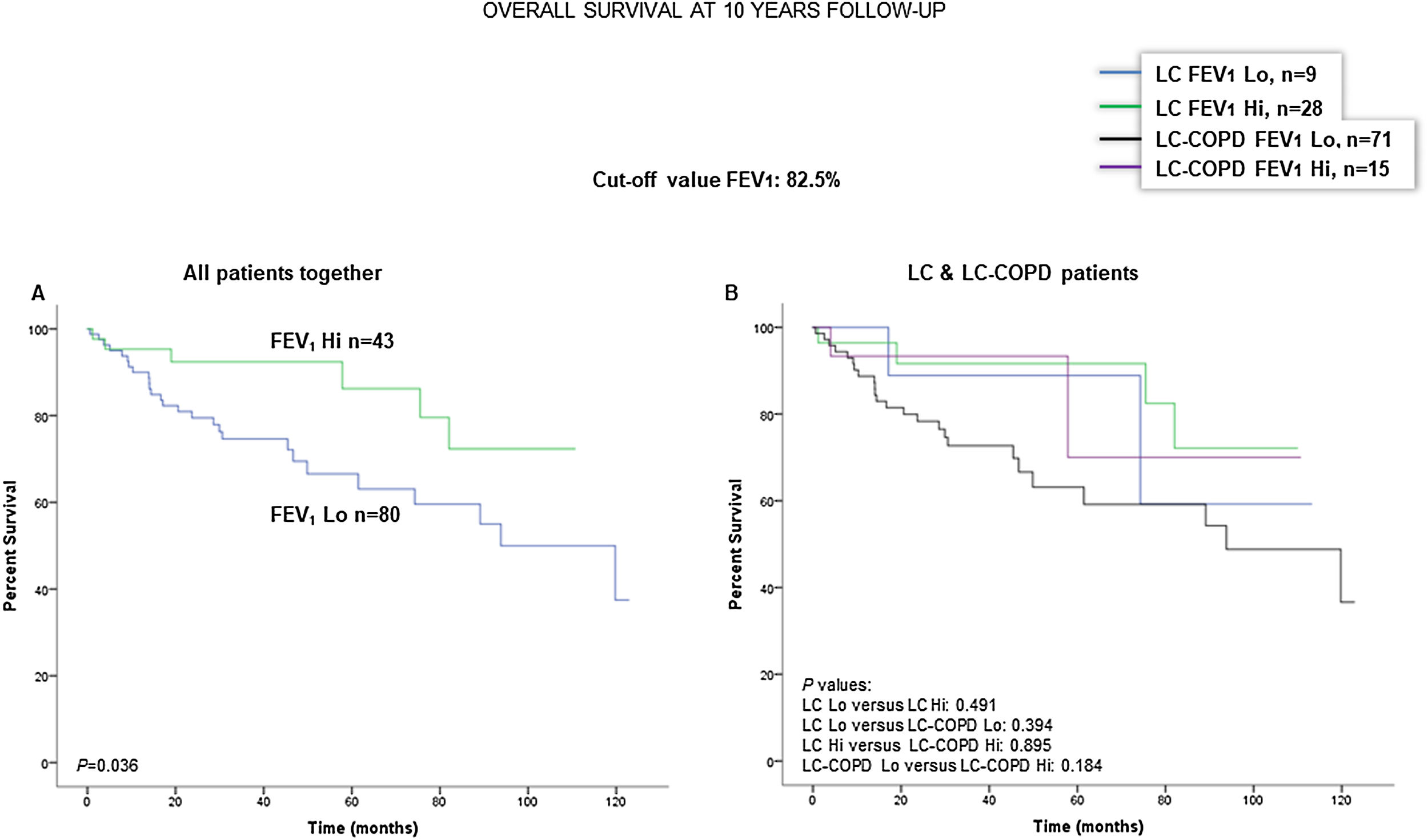
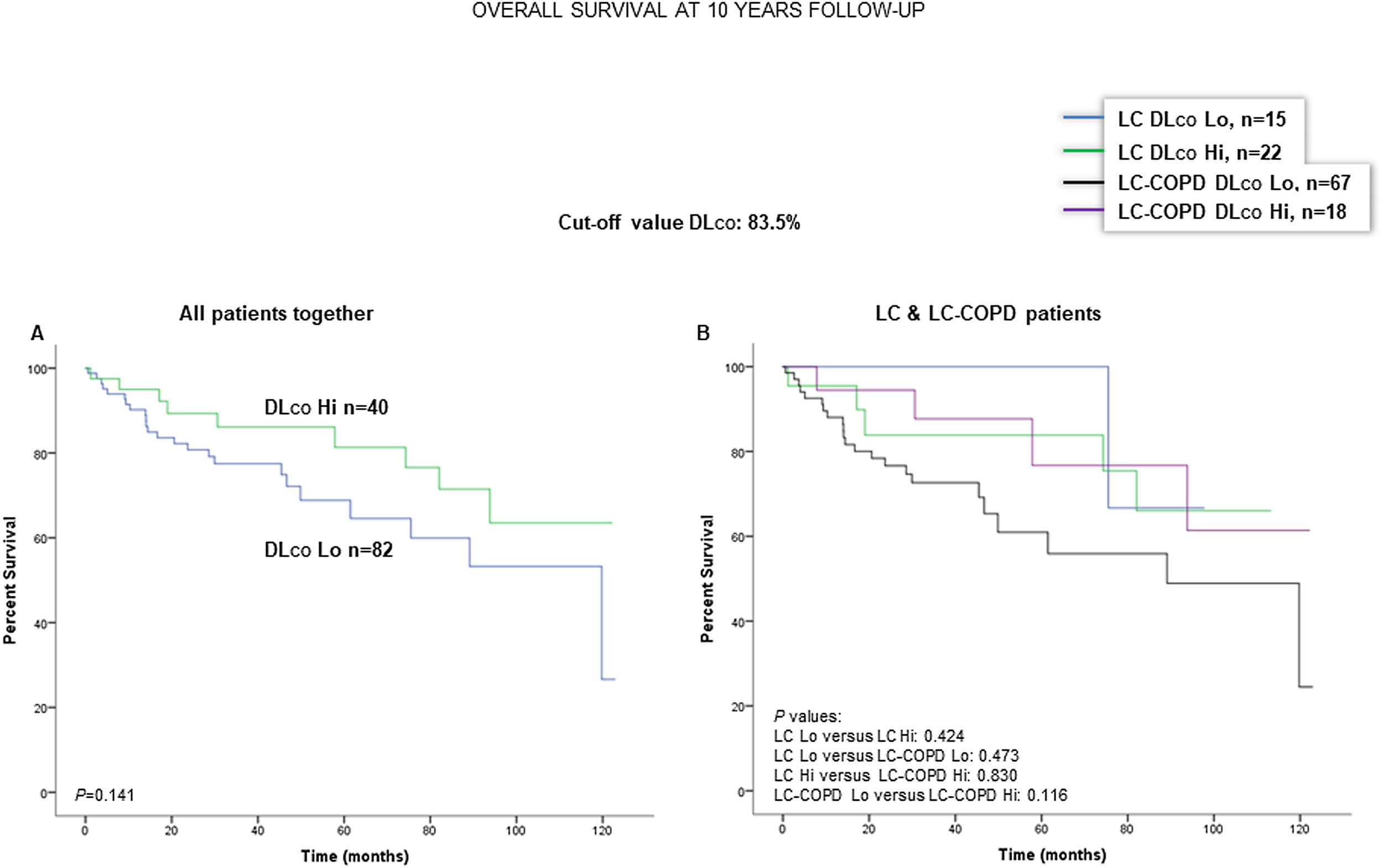
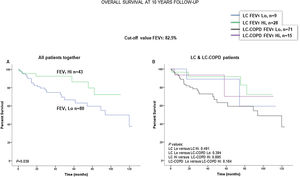
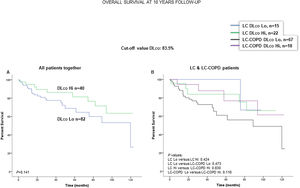
When all patients were analyzed together, a lower level of FEV1 (cut-off: 82.5%) was associated with a poorer 10-year survival (Fig. 6A). When patients were subdivided according to the presence of COPD, no significant differences in ten-year survival were observed in any of the subgroups (Fig. 6B). No significant differences were seen when non-smokers (never smokers and ex-smokers) were analyzed independently (Fig. S6A and S6B). When all patients were analyzed together, a lower level of DLCO (cut-off: 83.5%) was not significantly associated with a poorer 10-year survival (Fig. 7A). When patients were subdivided according to the presence of COPD, no significant differences were observed in any of the subgroups (Fig. 7B). No significant differences were seen when non-smokers (never smokers and ex-smokers) were analyzed separately (Fig. S7A and S7B).
(A) Kaplan–Meier survival curves for OS in all patients based on the cut-off value of FEV1 (above and below the cut-off value: 82.5%). (B) Kaplan–Meier survival curves for OS in LC patients with and without COPD based on the cut-off value of FEV1 (above and below the cut-off value: 82.5%). This information was not available in two patients. Definition of abbreviations: LC, lung cancer; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; Hi, high level (above cut-off value); Lo, low level (below cut-off value).
(A) Kaplan–Meier survival curves for OS in all patients based on the cut-off value of DLCO (above and below the cut-off value: 83.5%). (B) Kaplan–Meier survival curves for OS in LC patients with and without COPD based on the cut-off value of DLCO (above and below the cut-off value: 83.5%). This information was not available in three patients. Definition of abbreviations: LC, lung cancer; COPD, chronic obstructive pulmonary disease; DLCO, transfer factor of the lung for carbon monoxide; Hi, high level (above cut-off value); Lo, low level (below cut-off value).
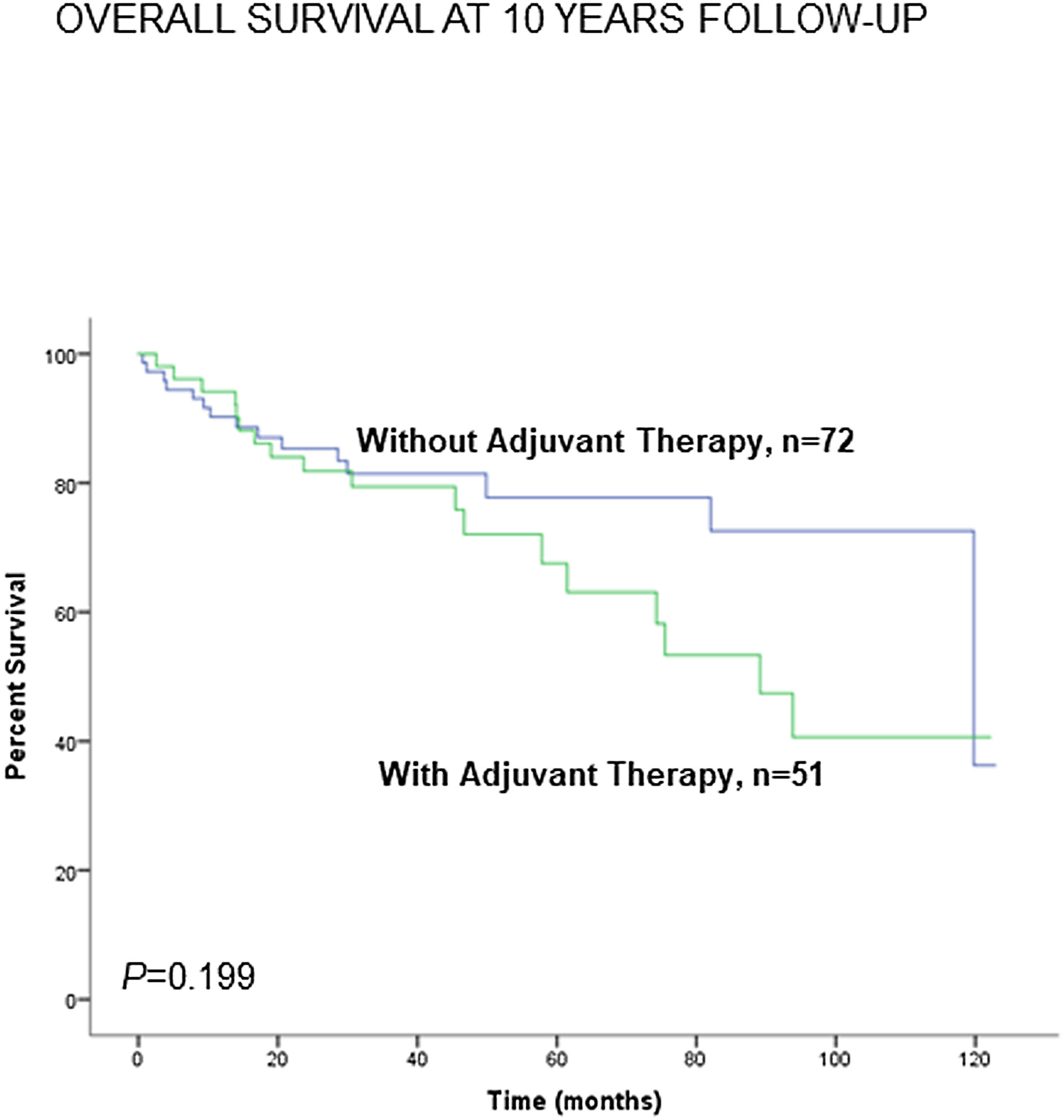
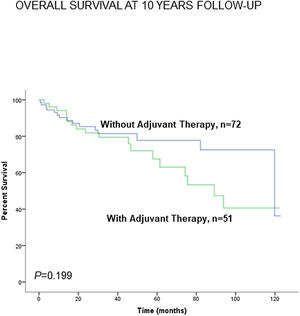
Administration of adjuvant therapy did not significantly influence the 10-year survival in any of the study groups of patients (Fig. 8). Consistently, no significant differences were seen when non-smokers (never-smokers and ex-smokers) were analyzed separately (Fig. S8).
Kaplan–Meier survival curves for OS in all patients based on the presence or absence of administration of adjuvant therapy to all patients together (LC and LC-COPD). This information was not available in two patients. Definition of abbreviations: LC, lung cancer; COPD, chronic obstructive pulmonary disease.
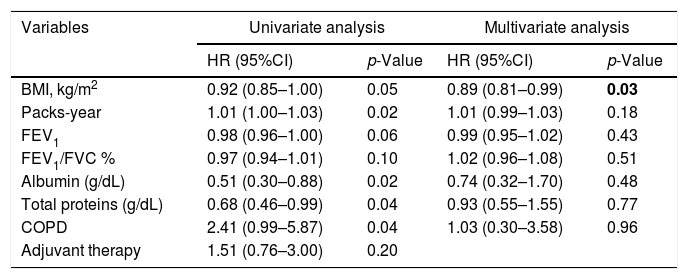
The univariate analysis showed that smoking history (HR=1.01, p=0.02), and COPD (HR=2.41, p=0.04) predicted a higher mortality risk, while BMI (HR=0.92, p=0.05), FEV1 (HR=0.98, p=0.06), albumin (HR=0.51, p=0.02), and total protein levels (HR=0.68, p=0.04) were associated with a lower mortality risk among all the LC patients (Table 2). Furthermore, the multivariate Cox proportional hazard regression analysis showed that BMI (HR=0.89, p=0.03) was an independent prognostic factor for OS among all the LC patients (Table 2).
Univariate and multivariate analyses with overall survival in all patients.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| BMI, kg/m2 | 0.92 (0.85–1.00) | 0.05 | 0.89 (0.81–0.99) | 0.03 |
| Packs-year | 1.01 (1.00–1.03) | 0.02 | 1.01 (0.99–1.03) | 0.18 |
| FEV1 | 0.98 (0.96–1.00) | 0.06 | 0.99 (0.95–1.02) | 0.43 |
| FEV1/FVC % | 0.97 (0.94–1.01) | 0.10 | 1.02 (0.96–1.08) | 0.51 |
| Albumin (g/dL) | 0.51 (0.30–0.88) | 0.02 | 0.74 (0.32–1.70) | 0.48 |
| Total proteins (g/dL) | 0.68 (0.46–0.99) | 0.04 | 0.93 (0.55–1.55) | 0.77 |
| COPD | 2.41 (0.99–5.87) | 0.04 | 1.03 (0.30–3.58) | 0.96 |
| Adjuvant therapy | 1.51 (0.76–3.00) | 0.20 | ||
Definition of abbreviations: BMI, body mass index; FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
In the current investigation, the main findings were that the preoperative nutritional variables BMI and albumin and total protein levels in LC patients with relatively well-preserved nutritional status predicted mortality throughout a 10-year follow-up period. Furthermore, in LC patients with underlying COPD, lower levels of those nutritional parameters, especially BMI and albumin, were associated with a poorer survival. These results lead to the concept that presurgical nutritional status, even if within normal ranges, may predict long-term survival in patients with resectable lung neoplasms, particularly in those with underlying mild-to-moderate COPD (GOLD stages I and II). Collectively, these are relevant clinical findings, which were observed in patients with resectable LC, thus implying that in more advanced stages of LC, the associations between nutritional abnormalities and OS might be even more blatant. Nonetheless, a recent investigation,13 demonstrated that BMI, weight loss, and sarcopenia had a negative impact on survival of patients with resected lung tumors, independently of their tumor stage.
In patients with advanced stage LC, multivariate analyses demonstrated that BMI≥21kg/m2 was a favorable predictor of survival, whereas inflammatory markers and age were associated with poorer survival.16 Malnutrition (BMI and albumin) was prevalent in patients with advanced LC stages and in general was associated with impaired clinical outcomes.17 The prevalence of postoperative complications was also associated with BMI in LC patients who underwent surgery for their neoplasm.18,19 In LC patients with underlying emphysema or fibrosis albumin was shown to predict OS at five years of follow-up.30
Other reports have also demonstrated the implications between nutritional parameters and long-term survival among patients with LC. For instance, a reduction in more than 5% BMI significantly increased all-cause mortality in a large cohort (81,388 cases) of patients with different cancer types including lung cancer.14 In another study,15 weight loss and low albumin levels also exerted a negative impact on disease survival even in patients receiving immunotherapy. In a meta-analysis,31 healthy life style habits were also associated with better prognosis for several cancer types including LC.
In the current investigation, the study patients with LC were further subdivided according to the presence of underlying COPD. Associations between several nutritional parameters measured prior to surgery and OS were found in all patients as a group, but particularly in those with COPD. These are novel findings that put the line forward that preoperative nutritional status, even if well-preserved, is a clinical feature that warrants attention in LC patients, particularly in those with airway obstruction. Importantly, variables such as BMI, albumin, and total protein levels on the one hand, and packs-year, FEV1, and the presence of COPD were independently associated with OS in the univariate analyses. Nonetheless, in the multivariate analyses, BMI was the only variable that kept the prognosis value among all the study patients. This is a novel finding that is in line with previous studies in which BMI was also associated with postoperative complications following surgery.18,19 Another novelty in the current investigation was that patients were followed up to a period of 10 years as opposed to studies where patients were followed up for shorter periods of time were retrospective.16–19,30
Importantly, despite the significant reduction in BMI and in the blood parameter albumin seen in the LC-COPD patients, their levels remained within the normal range for the most of the patients in both groups. These are relevant observations, since they could be ignored in standard clinical settings, while they have proved to have a prognostic value when patients were followed up for several years. This is even more important, given that the parameters were measured at baseline, prior to undergoing a major surgical procedure for the curative treatment of their lung neoplasms. To sum up, although BMI was within normal ranges or even high in the majority of the patients analyzed in this cohort, we believe that it should be carefully evaluated and considered as an important clinical, prognostic parameter in patients who have to undergo surgical procedures and when designing original research studies.
In general, LC patients with baseline impairment in body weight and/or muscle mass are the targets for nutritional support and follow-up. Commonly, patients with nutritional depletion are those exhibiting more advanced stages of their tumors including those with LC. It is likely that tumors induce a hypercatabolic state that renders patients more susceptible to experience weight loss and eventually to cachectic states.32 Nonetheless, baseline poor nutritional status and altered body weight and composition may, in turn, elicit deficiencies of the immune system activity that may favor the progress of tumor growth and development.33,34 Therefore, it is important that nutritional abnormalities and body weight loss, even though of small magnitude, are identified and diagnosed rapidly in clinical setting of LC patients, particularly in those with underlying respiratory conditions such as COPD. Furthermore, COPD per se was also shown to worsen disease prognosis among all patients with LC, suggesting that an additional chronic respiratory condition impaired OS in this cohort of patients. In the present investigation, however, COPD patients with LC did not experience significant differences in the variable weight loss quantified prior to surgery with respect to LC patients with no COPD. These findings reveal that BMI per se is a strong predictor of the LC patients’ survival irrespective of whether they experienced significant body weight loss prior to surgery.
Importantly, cigarette smoking above 48 packs-year was significantly associated with a worse prognosis among all patients with LC in the present cohort. Moreover, in LC with COPD an almost significant association with a worse OS was also observed. These are clinically relevant findings that are in accordance with recent results,35 in which cigarette smoking burden significantly correlated with survival in patients with lung adenocarcinoma. Nonetheless, in the multivariate analysis the influence of CS was lost. This is in line with the lack of differences in the subanalyses.
Study limitationsIn this cohort of LC patients with relatively well-preserved nutritional status, international nutritional classifications have not been used as only very few patients would have fell into the most severe categories. Furthermore, several parameters indispensable for the classification of the patients into several groups of nutritional abnormalities were not available for all of them.36,37
Another limitation might be the surgical procedure (thoracotomy versus VATS) used to resecting the lung tumors in the study patients. Nevertheless, we believe that this has had no significant effect on either BMI or albumin prior to surgery or on the long-term survival of the patients as also demonstrated previously.38,39
In the present investigation, no specific scales of comorbidity were used prior to patient recruitment.40 Nonetheless, very selective inclusion and exclusion criteria were established before patient recruitment. As described in methods, despite these limitations, we believe that the current investigation sheds light into the relevance of preoperative nutritional status, even if relatively well-preserved, in patients with resectable lung tumors and in particular in those with underlying COPD. The current results will serve as the basis for the design of multicenter studies to analyze the prognosis value of other variables using similar approaches in the near-future.
ConclusionsIn the present cohort of LC patients with resectable tumors and well-preserved nutritional status, the parameters BMI and blood albumin and protein levels measured at baseline prior to thoracotomy predicted OS, especially in those with underlying COPD. These are clinically relevant findings, since values of those nutritional parameters were within the normal ranges in the majority of the analyzed patients. A thorough nutritional preoperative assessment should be included in the study of patients with resectable LC, particularly in those with chronic airway obstruction.
Authors’ contributionsStudy conception and design: EB, VC; Patient assessment and recruitment: JT, VC, DRC; Surgical procedures and staging: ARF, RA; Statistical analyses and data interpretation: XD, JT, DRC, VC, EB; manuscript drafting and intellectual input: EB, JT; manuscript writing final version: EB.
Sources of fundingThis study has been supported by FIS 18/00075 & CIBERES (FEDER, Instituto de Salud Carlos III, Spain), SEPAR 2018, and unrestricted research grant from Menarini SA 2018.
Conflict of interestNone.
The authors are thankful to Ms Mireia Admetlló for her help with the patient clinical assessment.