Bronchopulmonary dysplasia (BPD) is the most common complication of extreme preterm delivery, and is associated with reduced exercise tolerance and exercise capacity. The aim of this study was to assess the effects of a physical activity programme on exercise tolerance, exercise capacity, flexibility, and lung function in prematurely born children with BPD.
MethodsThis was a randomized controlled trial. Preterm children with BPD (4–6 years) were randomized to intervention (IG) and control (CG) groups. The CG did not participate in any physical activity during the study period. The IG performed a 4-week exercise programme based on aerobic interval and resistance training. Outcomes were based on the 6-minute walk test (6MWT), incremental shuttle walk test (ISWT), modified sit and reach test (MSRT) and spirometry results.
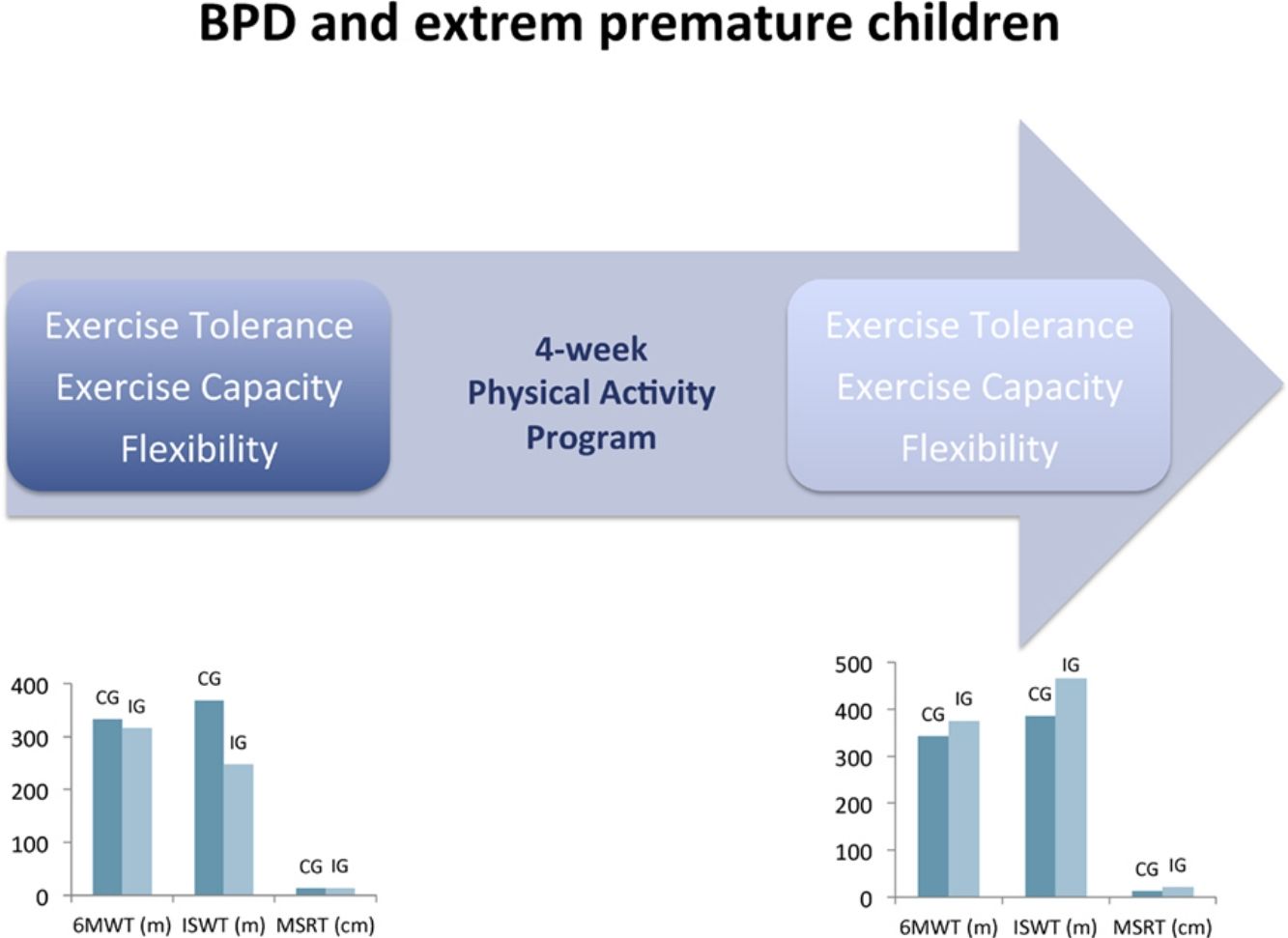
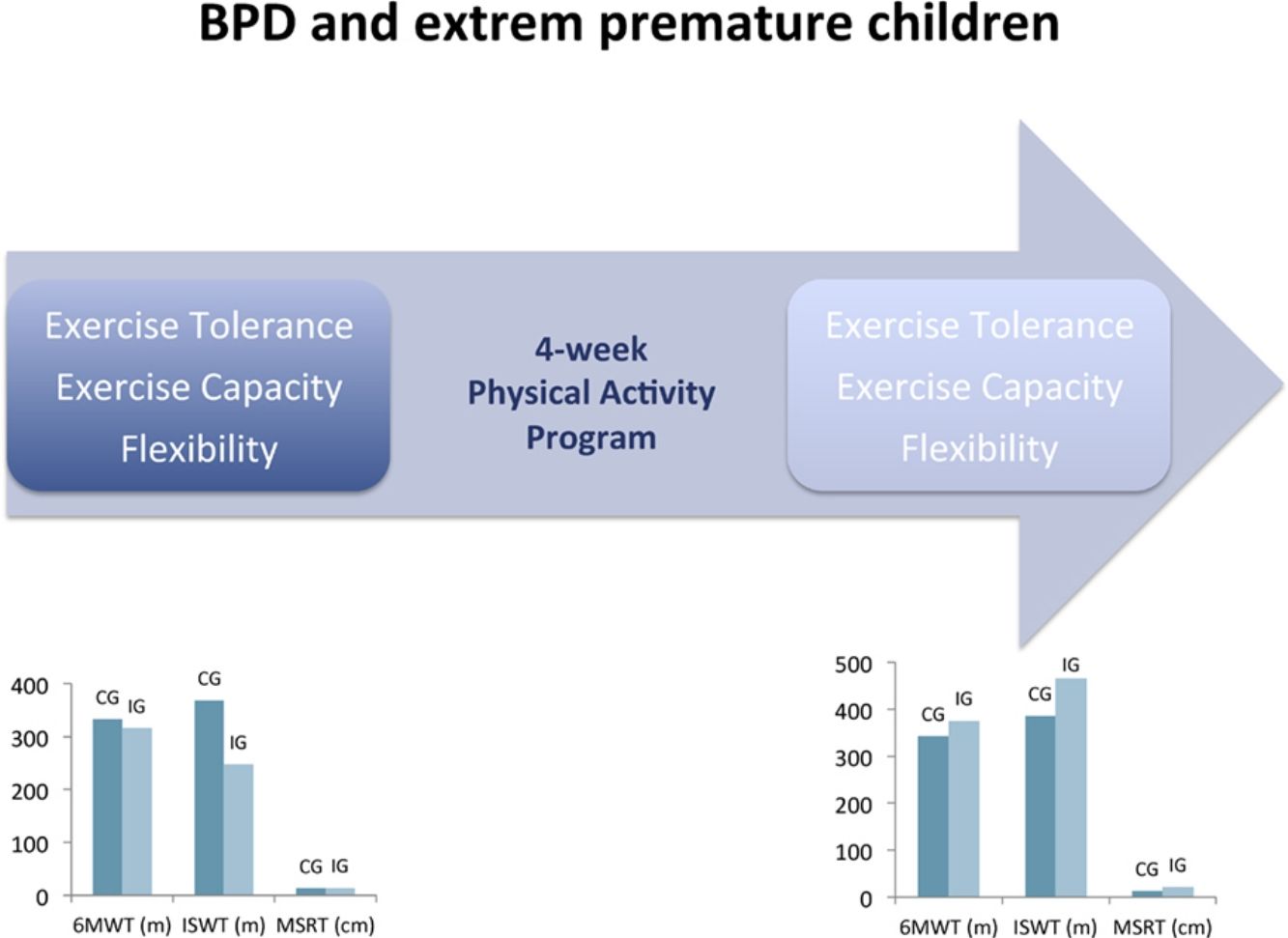
ResultsTwenty individuals were recruited. In the IG (n=10), statistical and clinical improvement was observed in the 6MWT (316.3±31.4m vs 376.2±39.5m; P=.002). Significant improvements were also seen in the IG in the ISWT (248.0±45.2m vs 465.3±58.2m; P=.013), MSRT (14.5±7.7cm vs 22.8±6.9cm; P=.003), and FEV1 (102%±16% pred vs 104%±17% pred; P=.004). No significant differences between pre- and post-intervention were observed in the CG for all outcomes (n=10).
ConclusionThis 4-week programme resulted in statistical and clinical improvements in exercise tolerance, exercise capacity and flexibility in preterm children with BPD.
La displasia broncopulmonar (DBP) es una secuela frecuente entre los prematuros extremos, asociándose a una reducción en la tolerancia y en la capacidad al ejercicio. El objetivo de este estudio es evaluar los efectos de un programa de entrenamiento basado en la tolerancia y en la capacidad al ejercicio, la flexibilidad y la función pulmonar en niños prematuros con DBP.
MétodosEl ensayo clínico se hizo con niños prematuros con DBP (de 4 a 6 años), aleatorizados en 2 grupos, control (GC) e intervención (GI). El GC no participó en ninguna actividad física durante el estudio. El GI realizó un programa interválico y de resistencia de 4 semanas. Se evaluó el Six Minute Walking test (6MWT), el Incremental Shuttle Walk test (ISWT), el Modified Sit and Reach test (MSRT) y la espirometría.
ResultadosSe reclutaron 20 niños. No se observaron diferencias significativas entre la pre- y la postintervención en el GC (n=10). En el 6MWT se observó una mejoría significativa y clínica (316,3±31,4m vs. 376,2±39,5m; p=0,002) al final de la intervención en el GI (n=10). El ISWT (248,0±45,2m vs. 465,3±58,2m; p=0,013), el MSRT (14,5±7,7cm vs. 22,8±6,9cm; p=0,003) y la FEV1 (102±16% pred vs. 104±17% pred; p=0,004) también mejoraron significativamente en el GI.
ConclusionesEste programa de 4 semanas, mejora estadísticamente y clínicamente la tolerancia y la capacidad al ejercicio, y la flexibilidad en niños prematuros con DBP.
Bronchopulmonary dysplasia (BPD) is the most frequent severe respiratory disorder during the neonatal period among premature children.1 In Europe, the incidence is approximately 20% among new born with low birth weight (BW) (<2500g) and 30% in extremely preterm (<28 week).2 BPD incidence is related to gestational age (GA) and low BW. Indeed, in Spain, it affects 53% of children who were born before 28 weeks of GA compared to only 0.3% of children with a GA greater than 30 weeks, and 67% of children with a BW lower than 800g compared to 1% of children with a BW around 1250–1500g.3
Extremely preterm infants and, particularly those with BPD, have frequently impaired lung function in infancy, childhood and adulthood. It increases the risk of respiratory disease. Reduced functional capacity was also demonstrated in BPD preterm children and adolescents compared to children born full-term.1,4–6 These children have an important risk of limited ventilatory reserve and/or a need for adaptation during exercise, both resulting in limitations in daily life.7
Exercises programme are highly recommended in many lung diseases.8,9 Indeed, in chronic obstructive pulmonary disease (COPD), pulmonary rehabilitation including a physical exercise program is highly effective (Evidence 1A)8. In paediatric chronic diseases such as congenital heart diseases or cystic fibrosis, exercise training was also recommended as an important part of the treatment.8 However, the effects of such programme have never been documented in BPD preterm children, despite similar physical limitations. We hypothesized that BPD preterm children would benefit from a physical activity programme.
The aim of this study was to evaluate the effects of a physical activity programme on functional capacity (exercise tolerance and exercise capacity) (main outcome), flexibility and lung function in BPD extremely preterm children.
Material and MethodsPatientsBPD preterm children were recruited from database of the paediatric pulmonology unit of Hospital Vall d’Hebron in Barcelona (Spain) between August and November 2015. Inclusion criteria were to be aged between 4 and 6 years with a gestational age lower than 37 weeks, moderate BPD according to National Institute of Child Health and Human Development (NICHD) (<30% oxygen at 36 weeks, at postmenstrual age or at discharge)10 and free of participation in sports or another exercise programme at school or in a specific club. Children with congenital malformations, neuromuscular disorders, gait dysfunction, severe visual or hearing impairment, difficulties in communication or in understanding orders, wheezing or acute pulmonary disease in the 4 weeks preceding the study, haemodynamic changes at rest defined by heart rate higher than 130bpm, systolic blood pressure higher than 130mmHg or diastolic blood pressure higher than 80mmHg and lung and/or heart transplantation were excluded.
The study was previously approved by the institutional medical ethics committee (PIC-140-15) and was registered in ClinicalTrial.gov (NCT02523521). All parents signed a written informed consent.
Design and ProgrammeThis was a randomized controlled trial. Allocation was concealed. Subjects were randomized in intervention (IG) or control group (CG). Randomization was performed by a computer-generated a random number list (http://www.randomizer.org/).
IG performed a twice-a-week specific and supervised outpatient group exercise programme at the hospital during 4 weeks. Each session was divided in 3 blocks: (1) interval training (duration from 30 to 40min) by running exercises, (2) peripheral muscle strength exercises (biceps, triceps, deltoids, hamstrings, quadriceps and triceps surae) with medicine ball from 0.5kg to 2kg (during 5–10min) and (3) peripheral muscles stretching (hamstrings, quadriceps, triceps surae, deltoids, biceps, pectoral and dorsal) and chest flexibility (side inclinations) (during 5–10min). The intensity of the interval training was determined by modified Borg scale (from 5 to 8 for moderate to vigorous intensity).11 The duration of the sessions increased every week from 30 to 50min. CG was evaluated but they did not participate to any specific physical activity during the study period.
Evaluation and OutcomesThe same qualified investigator (NM) performed all the evaluations and sessions and was blinded to the allocated treatment at the time of the initial evaluation. Lung function test was performed by a blinded pulmonologist. All the subjects were evaluated before and after the period of the study. The analysis was blinded.
Functional Exercise CapacityBased on the recent American Thoracic Society (ATS)/European Respiratory Society (ERS) statement,12 functional exercise capacity (exercise tolerance and exercise capacity) was considered as the main outcome and assessed by two different tests: six minute walking test (6MWT) and incremental shuttle walk test (ISWT).
Exercise tolerance was evaluated by the 6MWT, according to the standardized protocol validated by Li et al. in children13 and derived from ATS/ERS.14 No training test was carried out due to the high reproducibility of this test in children and the absence of learning effect.13,15 Children took a 10-min rest on a chair before the test. They were instructed to walk as far as possible for 6min. The test was self-paced and the child was allowed to rest if desired, although the clock continued to run. The encouragement during the test was standardized (“keep going”, “you are doing fine”, “everything is going well”) and given by the same person at fixed times.13 Walked distance was expressed as absolute and percentage of predicted value based on Geiger's equation (6MWD (m)=196.72+39.816×age (years)−1.36×age2 (years)+138.28×height (m)).16
Exercise capacity was measured by the ISWT, recently validated by Lanza et al. in children17 and based on Singh's protocol.18 No training test was performed due to it is high reproducibility.17 The test was performed in a 10-m hallway with 2 cones at the endpoints. Children were asked to walk (or ran) around the course at an incrementing pace directed by an audio signal.17 The initial walking speed was 0.5m/s and increased by 0.17m/s each minute. The speed increment was indicated by a triple bleep. The test was composed of 15 levels. Walked distance was calculated and expressed as absolute and percent of predicted value following Lanza's equation (ISWT (m)=845.559+(sex×193.265)+(age×47.850) (body mass index×26.179)).17 (Sex: 0 for female, 1 for male).
The child took a rest for 20min between 6MWT and ISWT. Pulsed oxygen saturation and heart rate were recorded before, after and 2min after each test (Nonin, Onyx, USA). The test was interrupted on request by the child due to exhaustion or in case of oxygen saturation lower than 80% or heart rate higher than 85% of the maximum heart rate for age [predicted maximum HR=210−(0.65×age)].19
FlexibilityFlexibility was assessed by the Modified Sit and Reach test (MSRT). Children were seated on the floor with both legs fully extended, shoulder width apart, and feet placed flat against a box with a slide ruler attached to the top. The height of the box was 33cm. With one hand on top of the other and the knees fully extended, they slowly reached forward (without jerking) as far away as possible, sliding the hands across the top of the ruler, and held the final position at least 2s. The score (in centimetres) was registered as the final position of the fingertips on or towards the ruler. MSRT score was calculated using the following equation: finger-to-box distance (cm)+(Sit and Reach Test (SRT) score−15cm).20 Higher scores indicated better performance. The reached distance of 15cm corresponded with the position of the feet against the box. The score was negative if the participant was not able to touch the front of the box with his fingertips. The MSRT was performed twice and the average value was retained.20 MSRT distance was calculated and expressed in absolute value.
Lung FunctionLung function test was performed according to ATS/ERS recommendations.21 Forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), ratio of forced expiratory volume in 1s to forced vital capacity (FEV1/FVC) and forced expiratory flow (FEF25%–75%) were evaluated. The results were expressed by Global Lung Initiative predicted value equation (GLI 2012).22
Statistical AnalysesThe sample size corresponding to a difference of 10% in the walking distance (main outcome), between the pre- and post-intervention at a 5% significance level for a 2-sided test with a power of 80% was determined (n=8 per group) based on Geiger et al. results.16 Clinical data were analyzed by SPSS Statistics 24.0 (IBM Corp., Armonk, NY, USA). Descriptive results were expressed as mean±standard deviation (SD) and confidence interval, or by median and minimum–maximum depending on the normality of the distribution. Normality of the distributions was verified by Kolmogorov–Smirnov test. The means of pre- and post-intervention values were compared by Student T test. Paired or unpaired Student T test were used for intra- or intergroup comparisons, respectively. A P value less than .05 were considered statistically significant.
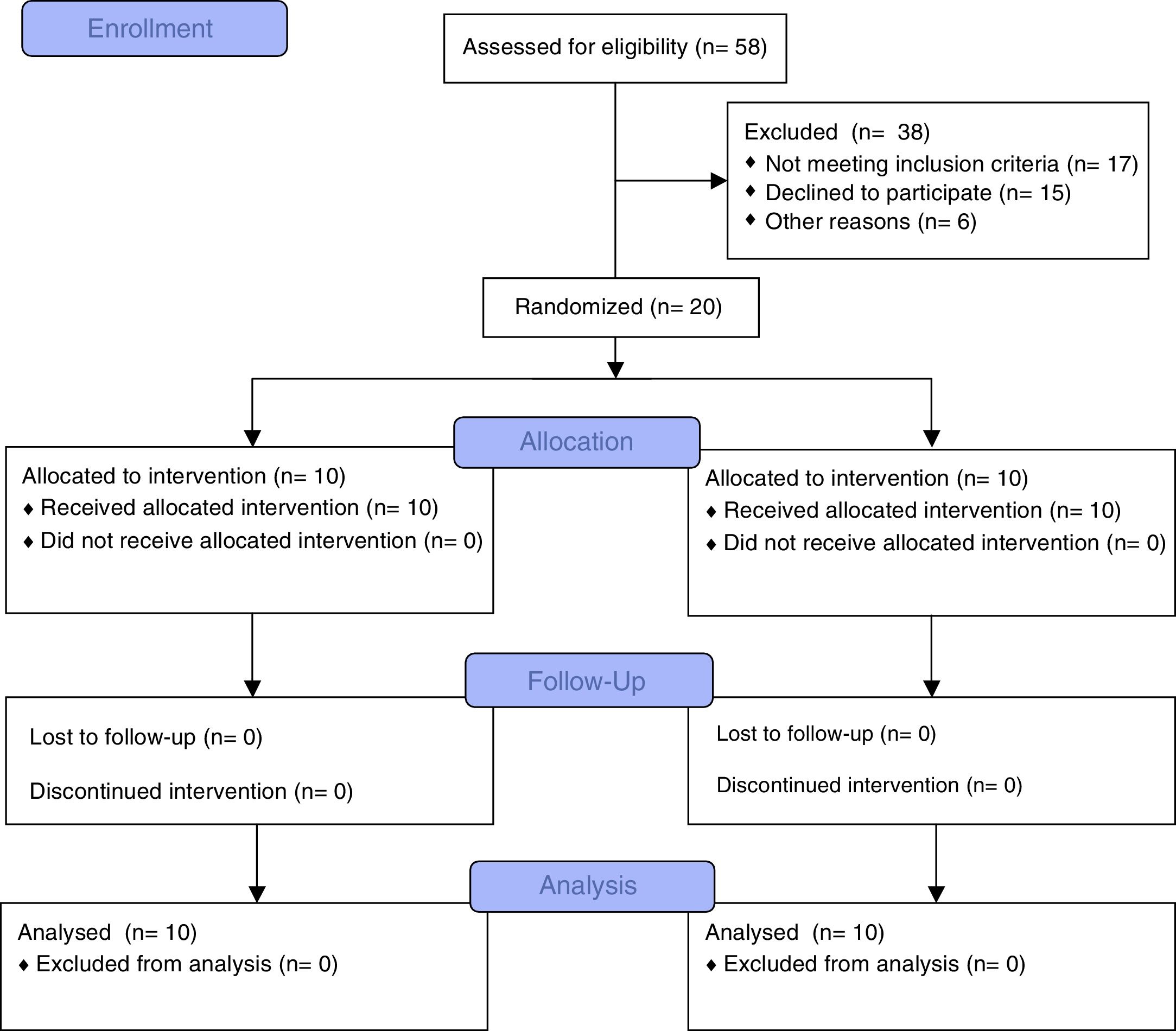
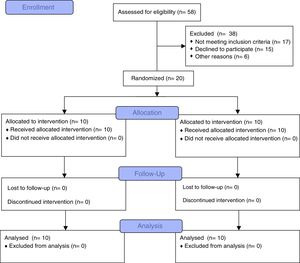
ResultsFifty-eight subjects (27 females, 31 males) were eligible. Twenty-six subjects fulfilled the inclusion criteria (gestational age=25.9±1.2 week, birth weight=747.2±131.5g). Six children were excluded due to lack of interest (n=2), travel (n=2) and language difficulties (n=2). Twenty children were randomized (6 females, 4 males in IG; 4 females, 6 males in CG) and completed the study (Fig. 1). No adverse events were observed neither during the evaluations nor during the programme.
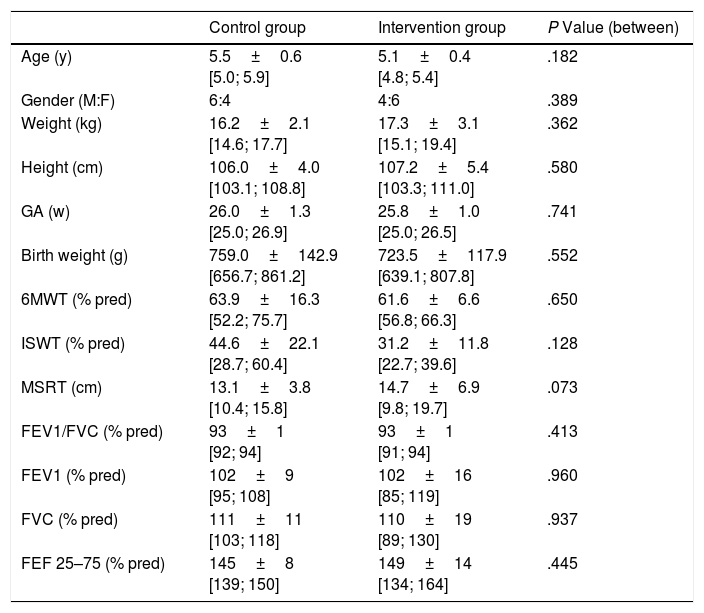
Characteristics of the subjects are presented in Table 1. Lung function was assessed in 10 and only 6 children in CG and IG respectively, due to the impossibility to obtain valid manoeuvres. Both groups were similar at inclusion for all analyzed outcomes (P>.05).
Characteristics of Included Children in Both Groups.
| Control group | Intervention group | P Value (between) | |
|---|---|---|---|
| Age (y) | 5.5±0.6 [5.0; 5.9] | 5.1±0.4 [4.8; 5.4] | .182 |
| Gender (M:F) | 6:4 | 4:6 | .389 |
| Weight (kg) | 16.2±2.1 [14.6; 17.7] | 17.3±3.1 [15.1; 19.4] | .362 |
| Height (cm) | 106.0±4.0 [103.1; 108.8] | 107.2±5.4 [103.3; 111.0] | .580 |
| GA (w) | 26.0±1.3 [25.0; 26.9] | 25.8±1.0 [25.0; 26.5] | .741 |
| Birth weight (g) | 759.0±142.9 [656.7; 861.2] | 723.5±117.9 [639.1; 807.8] | .552 |
| 6MWT (% pred) | 63.9±16.3 [52.2; 75.7] | 61.6±6.6 [56.8; 66.3] | .650 |
| ISWT (% pred) | 44.6±22.1 [28.7; 60.4] | 31.2±11.8 [22.7; 39.6] | .128 |
| MSRT (cm) | 13.1±3.8 [10.4; 15.8] | 14.7±6.9 [9.8; 19.7] | .073 |
| FEV1/FVC (% pred) | 93±1 [92; 94] | 93±1 [91; 94] | .413 |
| FEV1 (% pred) | 102±9 [95; 108] | 102±16 [85; 119] | .960 |
| FVC (% pred) | 111±11 [103; 118] | 110±19 [89; 130] | .937 |
| FEF 25–75 (% pred) | 145±8 [139; 150] | 149±14 [134; 164] | .445 |
GA: gestational age; y: years; M: male; F: female; kg: kilogram; g: grams; cm: centimetres; w: week; %: percentage; % pred: percentage of predicted value; 6MWT: six minute walk test; ISWT: incremental shuttle walk test; MSRT: modified sit and reach test; FEV1: forced expiratory volume in 1s; FVC: forced vital capacity; FEF 25%–75%: forced expiratory flow at 25%–75% of forced vital capacity.
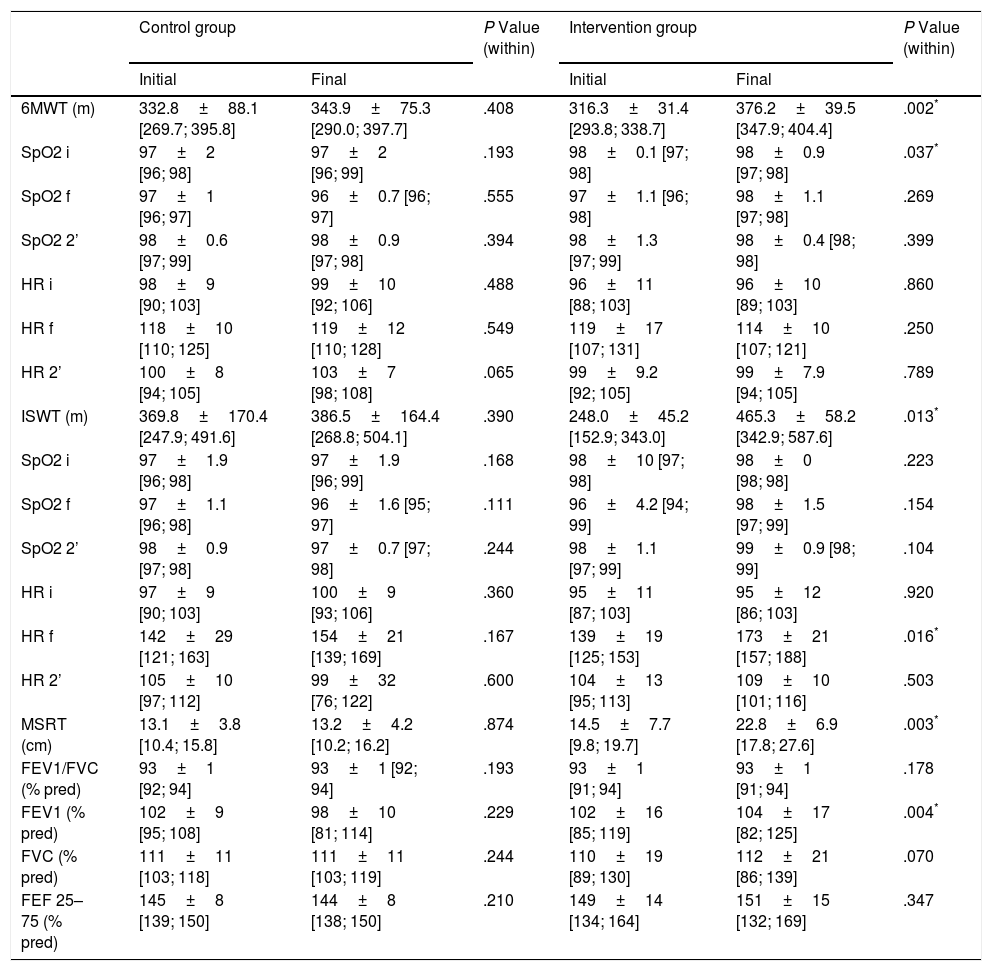
Effects of the intervention are illustrated in Table 2. After the 4-week programme, exercise tolerance (P=.002), exercise capacity (P=.013), flexibility (P=.003) and airways obstruction (P=.004) were significantly improved in the IG. No significant difference was observed for all outcomes between pre- and post-intervention in CG.
Comparison Between Pre- and Post-intervention in Both Groups.
| Control group | P Value (within) | Intervention group | P Value (within) | |||
|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | |||
| 6MWT (m) | 332.8±88.1 [269.7; 395.8] | 343.9±75.3 [290.0; 397.7] | .408 | 316.3±31.4 [293.8; 338.7] | 376.2±39.5 [347.9; 404.4] | .002* |
| SpO2 i | 97±2 [96; 98] | 97±2 [96; 99] | .193 | 98±0.1 [97; 98] | 98±0.9 [97; 98] | .037* |
| SpO2 f | 97±1 [96; 97] | 96±0.7 [96; 97] | .555 | 97±1.1 [96; 98] | 98±1.1 [97; 98] | .269 |
| SpO2 2’ | 98±0.6 [97; 99] | 98±0.9 [97; 98] | .394 | 98±1.3 [97; 99] | 98±0.4 [98; 98] | .399 |
| HR i | 98±9 [90; 103] | 99±10 [92; 106] | .488 | 96±11 [88; 103] | 96±10 [89; 103] | .860 |
| HR f | 118±10 [110; 125] | 119±12 [110; 128] | .549 | 119±17 [107; 131] | 114±10 [107; 121] | .250 |
| HR 2’ | 100±8 [94; 105] | 103±7 [98; 108] | .065 | 99±9.2 [92; 105] | 99±7.9 [94; 105] | .789 |
| ISWT (m) | 369.8±170.4 [247.9; 491.6] | 386.5±164.4 [268.8; 504.1] | .390 | 248.0±45.2 [152.9; 343.0] | 465.3±58.2 [342.9; 587.6] | .013* |
| SpO2 i | 97±1.9 [96; 98] | 97±1.9 [96; 99] | .168 | 98±10 [97; 98] | 98±0 [98; 98] | .223 |
| SpO2 f | 97±1.1 [96; 98] | 96±1.6 [95; 97] | .111 | 96±4.2 [94; 99] | 98±1.5 [97; 99] | .154 |
| SpO2 2’ | 98±0.9 [97; 98] | 97±0.7 [97; 98] | .244 | 98±1.1 [97; 99] | 99±0.9 [98; 99] | .104 |
| HR i | 97±9 [90; 103] | 100±9 [93; 106] | .360 | 95±11 [87; 103] | 95±12 [86; 103] | .920 |
| HR f | 142±29 [121; 163] | 154±21 [139; 169] | .167 | 139±19 [125; 153] | 173±21 [157; 188] | .016* |
| HR 2’ | 105±10 [97; 112] | 99±32 [76; 122] | .600 | 104±13 [95; 113] | 109±10 [101; 116] | .503 |
| MSRT (cm) | 13.1±3.8 [10.4; 15.8] | 13.2±4.2 [10.2; 16.2] | .874 | 14.5±7.7 [9.8; 19.7] | 22.8±6.9 [17.8; 27.6] | .003* |
| FEV1/FVC (% pred) | 93±1 [92; 94] | 93±1 [92; 94] | .193 | 93±1 [91; 94] | 93±1 [91; 94] | .178 |
| FEV1 (% pred) | 102±9 [95; 108] | 98±10 [81; 114] | .229 | 102±16 [85; 119] | 104±17 [82; 125] | .004* |
| FVC (% pred) | 111±11 [103; 118] | 111±11 [103; 119] | .244 | 110±19 [89; 130] | 112±21 [86; 139] | .070 |
| FEF 25–75 (% pred) | 145±8 [139; 150] | 144±8 [138; 150] | .210 | 149±14 [134; 164] | 151±15 [132; 169] | .347 |
CG: control group; IG: intervention group; 6MWT: six minute walking test; ISWT: Incremental Shuttle Walk Test; MSRT: Modified Sit and Reach test; m: metres; cm: centimetres; % pred: percentage of predicted value; SpO2 i: initial oxygen saturation; SpO2 f: final oxygen saturation; SpO2 2’: two minutes after oxygen saturation measure; HR i: initial heart rate; HR f: final heart rate; HR 2’: two minutes after heart rate measure; FEV1: forced expiratory volume in 1s; FVC: forced vital capacity; FEF 25%–75%: forced expiratory flow at 25%–75% of forced vital capacity.
Cardiorespiratory response (pulsed oxygen saturation (SpO2) and heart rate (HR)) during 6MWT and ISWT are shown in Table 2. SpO2 did not show clinically relevant changes. HR at the end of ISWT was higher after the programme in IG (P=.016).
Lung function was similar at baseline in both groups. No changes in lung function were observed in CG. However, airways obstruction was significantly improved in IG (P=.004).
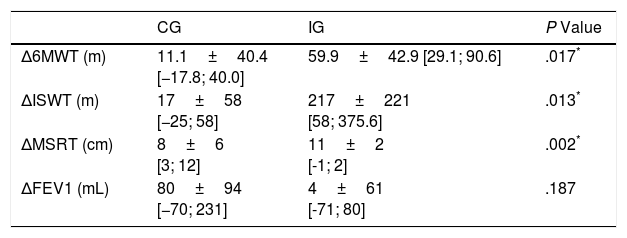
The improvement in 6MWT (gain in metres) (P=.017), ISWT (gain in metres) (P=.013) and MSRT (gain in centimetres) (P=.002) were significantly higher in IG than in CG and clinically relevant only in IG at the end of the programme (Table 3). It means that the improvement was higher than minimal clinically important difference (MCID) for these outcomes (15m for 6MWT, 40m for ISWT, 2.2cm for MSRT).
Comparison of Improvement Between Initial and Final Evaluations in Both Groups.
| CG | IG | P Value | |
|---|---|---|---|
| Δ6MWT (m) | 11.1±40.4 [−17.8; 40.0] | 59.9±42.9 [29.1; 90.6] | .017* |
| ΔISWT (m) | 17±58 [−25; 58] | 217±221 [58; 375.6] | .013* |
| ΔMSRT (cm) | 8±6 [3; 12] | 11±2 [-1; 2] | .002* |
| ΔFEV1 (mL) | 80±94 [−70; 231] | 4±61 [-71; 80] | .187 |
CG: control group; IG: intervention group; Δ: difference; 6MWT: six minute walking test; ISWT: Incremental Shuttle Walk Test; MSRT: Modified Sit and Reach test; m: metres; cm: centimetres; mL: millilitres.
The aim of this study was to evaluate the effect of a physical activity programme on functional exercise capacity (exercise tolerance and exercise capacity) (main outcome), flexibility and lung function in BPD extremely premature children (from 4 to 6 years old). After a 4-week exercise programme, we highlighted a clinically relevant and statistically significant improvement in functional exercise capacity and flexibility. Airway obstruction level was also improved even if it was not clinically relevant.
Functional exercise capacity was considered as the main outcome. The ATS/ERS propose assessing this endpoint by two different validated, inexpensive and safe tests (6MWT and ISWT).12 6MWT has been shown to reflect daily life activities limitation,23 to measure the response to therapeutic interventions and to be strongly correlated to cardiopulmonary exercise test.24 ISWT is incremental and progressive test, stressing the individual symptom limiting maximal performance.18
In adults, it is well know that a learning effect exists in field tests (6MWT and ISWT).12 Then, to decrease this effect a training test has to be performed.14 However, Martins et al. evaluated the repeatability of the 6MWT in children with 30min between both tests and they observed no significant difference in the walked distance between both tests.15 They suggested that there is no learning effect in children. Li demonstrated also a good reproducibility in children with no significant difference in the walked distance when 6MWT was repeated after 18 days.13 This study concerned non-Caucasian children but we postulated that the learning effect (or reproducibility) on walking distance is not related to the ethnic origin. For these reasons, we did not perform a training test.
Our programme improved functional exercise capacity in the recruited children. As previously demonstrated,1,4,5,25,26 BPD children showed lower functional exercise capacity compared to healthy children. Indeed, in our study, walked distance in 6MWT was only 61% and 64% of the predicted values at baseline in IG and CG, respectively.16 Spanish children have one of the most sedentary behaviour in Europe27 and it can contribute to the reduced walked distance. We hypothesize that such a programme could reduce the risk of sedentary and inactivity associated with being born extremely prematurely. After the exercise programme, the distance improved only in IG (+60m). It reinforces this hypothesis. Despite MCID has never been determined for 6MWT in children, this improvement can be considered as clinically relevant. Indeed, it was largely higher than the error related to the repeatability (15m) of this test in children.13 The distance walked during ISWT was also dramatically lower in both groups than in healthy children (45% and 31% of predicted value in CG and IG respectively). IG improved significantly the walked distance (217m) at the end of the programme. This improvement was clinically relevant.
Flexibility is a health-related fitness quality included in several fitness batteries.28 The most used evaluation tools are the SRT and the MSRT. SRT is validated for estimating hamstring extensibility in children.29 However, MSRT protocol decreases the limb length bias establishing a relative zero point for each person. Even if Hoeger et al. demonstrated no significant differences between SRT and MSRT,30 it can be particularly important in paediatric population to perform MSRT due to anthropometric characteristics of children. After our 4-week programme, we observed a significant improvement (+8.3cm) in flexibility in IG (14.5±7.7 vs 22.8±6.9; P=.003). De Miguel-Etayo et al.31 proposed European reference values of pre-pubertal children of fitness measures (6–11 years-old). Comparing their 6-years age group (19.8cm in boys and 21.9cm in girls) with our baseline values, we found a reduced flexibility among BPD children in CG (14.8cm in boys and 12.0cm in girls) and IG (12.1cm in boys and 16.6cm in girls). Mayorga-Vega et al.32 considered 2.2cm as a minimal detectable change value of the SRT. In our study, we found a greater improvement in IG (+6.1cm). As previously demonstrated,33 our results corroborated that extremely low birth weight (ELBW) BPD children have less flexibility compared to term-born children (P=.002).
Cardiorespiratory response during exercise are similar between preterm and full-term children5,34 and between preterm children with and without BPD.4 As expected, in our study, this response at exercise (SpO2 and HR) was normal in all included children.
In general, lung function tends to be reduced in BPD children. Contrary to previous publications,4,26,35 it was normal in both groups at inclusion. It can be explained by the impairment in lung function related to different BPD definitions,36 the BPD severity37 and the choice of Global Lung Function Initiative equation as reference values.22 BPD children tend to present airway obstruction, especially in adolescence and adulthood9,35,38,39 and their lung function may deteriorate at a more rapid rate compared to healthy children.36 For this reason, Rosenfeld40 suggested that spirometry provides a useful longitudinal measurement in young children with BPD. Despite that, there is a paucity of data on preschool spirometry in children with BPD. Spirometric tests can be performed in children from 3 years.22 Despite the difficulty to perform lung function test in preschool children (4–6 years old), only four children from our sample failed to perform successful spirometry manoeuvres in our study. A similar success rate was classically observed in children under 6 years old.41,42 Surprisingly to what it is classically expected after exercises programme, we observed a significant difference in FEV1 in IG (P=.004). However, this improvement is clinically non-relevant (lower than 8% predicted value or 150mL).43 It can be explained by the gain in peripheral and respiratory muscle strength associated to exercise training44 even if we did not evaluate them. Indeed, a relationship between FEV1 and peripheral45 and respiratory46 muscles strength was previously found in cystic fibrosis children.
To the best of our knowledge, no specific exercise programme has been proposed for BPD preterm children. This is the first study evaluating the effects of a short and supervised outpatient exercise programme in these patients. This programme included all the elements classically associated to exercise training programme in other diseases such as endurance (aerobic training), muscular strength training (resistance) and flexibility.8 Each week the intensity of the exercises and duration of the sessions were increased. Programme duration, structure, sessions duration and frequency, and training intensity were extrapolated and adapted from pulmonary rehabilitation programme for COPD and Cystic Fibrosis (CF) patients.8,47,48 We performed 2 sessions per week and the duration of each session varied from 40 to 60min based on ATS/ERS statement requiring a minimum of 30min.8 Our study confirmed the feasibility and the efficacy of such a programme in BPD children. Based on our results, we postulate that this programme is sufficient to obtain a benefit even though longer programme should be also considered in the future.
Some limitations need to be addressed. Even if the sample size was previously determined, extrapolation to larger population should be verified. We investigated only ELBW children with moderate BPD and we cannot extrapolate our results to less premature children. Children with neurocognitive sequels were excluded while they are usually associated with more severe BPD.49 However, it is the first time that a specific programme was proposed to BPD children. The duration of our programme was short. Indeed, the general recommendations consider that exercise programmes should be proposed during 6–12 week and 2–3 times per week.8,48 Nevertheless, similar 4-week programmes were already proposed with success in the literature.48,50,51
ConclusionsWe highlighted for the first time that a 4-week exercise programme was beneficial for BPD preterm children. This intervention at early age is feasible, leads to an improved exercise capacity. Indeed, our supervised programme improves exercise tolerance, exercise capacity and flexibility. As for all rehabilitation programmes, long-term benefit must be determined. Further studies will be necessary to evaluate the maintenance of the observed benefits and if children change their behaviour.
Funding StatementThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
AuthorshipNM and GR conceptualized and designed the study, drafted the initial manuscript, and approved the final manuscript as submitted.
AP, VM and GC carried out the initial analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Conflict of InterestsNo conflicts of interest.