Chronic obstructive pulmonary disease (COPD) is a major cause of death worldwide and although mortality from this disease is decreasing, it continues to cause millions of deaths yearly.1 Knowledge about COPD is primarily derived from observations centered on cigarette smoking, an important cause of the disease in all areas of the world. This editorial reviews what do these patients die from, the relevance of exacerbations or “chest attacks” on patient's outcomes and whether pharmacotherapy impacts on COPD mortality.
Dying From or With COPDDivo and coworkers2 have shown that COPD patients develop specific comorbidities that independently increase the risk of death, particularly coronary artery disease and lung cancer, which are the big mortality drivers in milder stages of COPD. This should direct health care providers to search for these diseases and manage them appropriately. The implementation of CT screening for lung cancer is one such measure that helps increase resectable tumor detection.3 As the severity of airflow limitation increases, the causes of death shift toward more respiratory causes, including pneumonia and respiratory insufficiency. In these patients, evaluation of lung function with lung volumes, functional capacity with the 6min walk distance, oximetry and/or arterial blood gases can help detect individuals who may benefit from pulmonary rehabilitation, supplemental oxygen or non-invasive mechanical ventilation.
Exacerbation or the “Chest Attack”Exacerbations or “Chest Attacks” are crucial events in the natural course of COPD. Although they can occur at any severity stage, their impact increases as patients develop more severe airflow limitation. Soler-Cataluna et al.4 first described the relationship between the presence and frequency of exacerbations with mortality. We now know that the first event increases the tempo and severity of subsequent events that impact on patient's outcomes. This was shown by Cote and co-workers5 who followed 205 patients over 2 years and observed that the BODE index (a surrogate marker of increased mortality risk) in patients who exacerbated, increased after the attacks and did not return to normal during the observation period. This was confirmed by Kunisaki et al. in a trial of over 16 thousand patients.6 The authors found that the hazard ratio (HR) for cardiovascular events was increased, particularly in the first 30 days after the attack (HR, 3.8; 95% CI, 2.7–5.5). The 30-day HR after a hospitalization for the episode was more than twofold greater (HR, 9.9; 95% CI, 6.6–14.9). The numbers of cardiovascular events occurring after the exacerbations suggests some vascular dysfunction leading to myocardial infarction, stroke and pulmonary embolism. It is clear that prevention of exacerbations is an important goal of therapy, if we are to decrease mortality in COPD. It may not be wise to wait for exacerbations to occur in order to begin prevention. In an analogy to coronary artery disease, it makes no sense to wait for the first heart attack before starting therapy for the disease. In this context, every effort has to be made to help patients stop smoking, institute appropriate vaccinations and maintain a healthy exercise program because all of these decrease exacerbations risk.
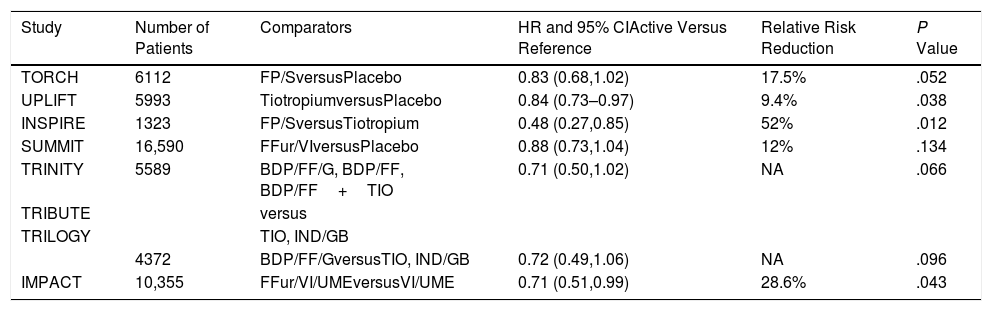
Pharmacotherapy and MortalityThe results of several large trials prove that pharmacotherapy reduces the risk of death in COPD. Let us start with the TORCH study, which randomized over 6000 patients for 3 years to four therapeutic arms; the active arm combined twice daily fluticasone proprionate/salmeterol (an ICS/LABA) and compared it with placebo, with two complementary arms corresponding to the individual components.7 There was a 17.5% relative risk reduction of mortality in the active arm compared with placebo, but the adjusted P value (due to an interim analysis that forced the statistical adjustment) was .052 and the study was unjustifiably seen as negative (Table 1). The subsequent SUMMIT trial that included over 16 thousand patients with moderate COPD (>60% predicted FEV1) randomized them to either once daily fluticasone furoate/vilanterol (ICS/LABA) or placebo and two arms that included the individual components.8 In this study, there was a 12% relative reduction in mortality risk between the active arm and the placebo (P=.137) and the results were again considered negative. There have been other studies with mortality included as a secondary outcome that further support the argument that pharmacotherapy decreases risk of death. The first was the 4 year UPLIFT trial, which recruited over 6000 patients randomized to receive the once daily LAMA tiotropium versus placebo.9 A statistical significance was observed at the end of the protocol-defined treatment period (P=.034) but not 30 days thereafter (P=.086). The hazard ratios for lower respiratory and cardiac mortality during treatment were 0.86 (95% CI 0.68–1.09) and 0.86 (95% CI 0.75–0.99) respectively. Another smaller study, the INSPIRE trial compared fluticasone Propionate/Salmeterol with Tiotropium and reported a 53% relative reduction in favor of the ICS/LABA.10 More recently, a stratified safety pooled analysis of all fatal events was performed comparing extrafine ICS-containing combinations versus ICS-free treatments in three 52-week studies (Tribute, Trinity and Trilogy).11 The comparisons included, triple with beclomethasone/formoterol/glycopyrronium compared with tiotropium, the same versus a combination of indacaterol/glycopyrronium and another with beclomethasone/formoterol. The analysis showed a reduction in the risk of death with a HR of 0.71 (95% CI 0.50–1.02; P=.066). Finally, the results of the IMPACT trial further supported this benefit.12 This 10,000 patients study compared triple therapy including fluticasone furoate/vilanterol/umeclidinium (ICS/LABA/LAMA) once daily, to one arm containing the same ICS/LABA and another the same LABA/LAMA. At study's end there was a relative risk reduction of death of 42% between the triple therapy and the LABA/LAMA combination and of 39% between the ICS/LABA and the LABA/LAMA. There was a non-significant relative reduction of 10.3% between triple and ICS/LABA. In the same studies, there were improvements in lung function, dyspnea, health status and exacerbations, including hospitalizations.
Summary of Results of Randomized Trials of Different Inhaled Therapies Where All-cause Mortality Was Either the Primary or a Secondary Outcome. Only the Primary Comparisons Are Shown for Those Studies Where There Were More Than 2 Arms.
| Study | Number of Patients | Comparators | HR and 95% CIActive Versus Reference | Relative Risk Reduction | P Value |
|---|---|---|---|---|---|
| TORCH | 6112 | FP/SversusPlacebo | 0.83 (0.68,1.02) | 17.5% | .052 |
| UPLIFT | 5993 | TiotropiumversusPlacebo | 0.84 (0.73–0.97) | 9.4% | .038 |
| INSPIRE | 1323 | FP/SversusTiotropium | 0.48 (0.27,0.85) | 52% | .012 |
| SUMMIT | 16,590 | FFur/VIversusPlacebo | 0.88 (0.73,1.04) | 12% | .134 |
| TRINITY | 5589 | BDP/FF/G, BDP/FF, BDP/FF+TIO | 0.71 (0.50,1.02) | NA | .066 |
| TRIBUTE | versus | ||||
| TRILOGY | TIO, IND/GB | ||||
| 4372 | BDP/FF/GversusTIO, IND/GB | 0.72 (0.49,1.06) | NA | .096 | |
| IMPACT | 10,355 | FFur/VI/UMEversusVI/UME | 0.71 (0.51,0.99) | 28.6% | .043 |
FP: fluticasone propionate; S: salmeterol. BDP: beclometasone dipropionate; FF: formoterol fumarate; G: glycopyrronium; TIO: tiotropium; IND: indacaterol; GB: glycopyrronium bromide; FFur: fluticasone furoate; VI: vilanterol UME: umeclidinium.
TORCH, Toward a Revolution in COPD Health.
UPLIFT, Understanding Potential Long-Term Impacts on Function with Tiotropium.
INSPIRE, Investigating New Standards for Prophylaxis in Reducing Exacerbations.
SUMMIT, Study to Understand Mortality and Morbidity in COPD.
IMPACT, Informing the Pathway of COPD Treatment.
With this evidence, it is not justified to maintain a nihilistic attitude about the effectiveness of the pharmacological agents currently available to our patients. Perhaps the decline in COPD deaths worldwide, is not only the consequence of altered smoking habits, but also of the judicious use of pharmacotherapy.