The development that bronchoscopy has experienced in the recent years has been spectacular, especially in the field of pediatrics. Both the diagnostic and the therapeutic applications of bronchoscopy have increased considerably. This expansion has been mainly based on the technological developments in various areas, such as instrumentation, optics, fiber optics, light sources, video-electronics and anesthesia techniques, to name just an important few.
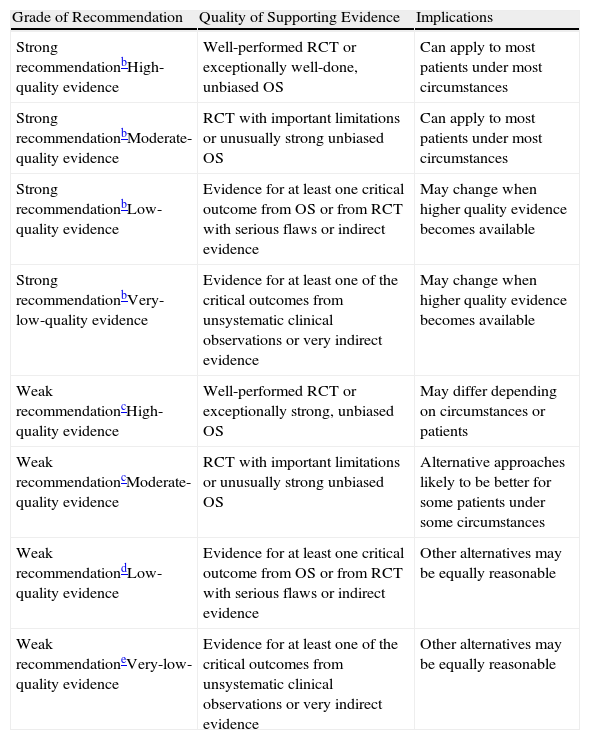
The aim of these guidelines is to improve, facilitate and unify the process of bronchoscopy in pediatrics. The recommendations have been established in keeping with the GRADE system1 (Table 1). In the aspects in which the scientific evidence is insufficient, we have included the recommendations agreed upon by consensus of the authors.
Classification of the Recommendations and Quality of the Evidence According to the GRADE System.a
| Grade of Recommendation | Quality of Supporting Evidence | Implications |
| Strong recommendationbHigh-quality evidence | Well-performed RCT or exceptionally well-done, unbiased OS | Can apply to most patients under most circumstances |
| Strong recommendationbModerate-quality evidence | RCT with important limitations or unusually strong unbiased OS | Can apply to most patients under most circumstances |
| Strong recommendationbLow-quality evidence | Evidence for at least one critical outcome from OS or from RCT with serious flaws or indirect evidence | May change when higher quality evidence becomes available |
| Strong recommendationbVery-low-quality evidence | Evidence for at least one of the critical outcomes from unsystematic clinical observations or very indirect evidence | May change when higher quality evidence becomes available |
| Weak recommendationcHigh-quality evidence | Well-performed RCT or exceptionally strong, unbiased OS | May differ depending on circumstances or patients |
| Weak recommendationcModerate-quality evidence | RCT with important limitations or unusually strong unbiased OS | Alternative approaches likely to be better for some patients under some circumstances |
| Weak recommendationdLow-quality evidence | Evidence for at least one critical outcome from OS or from RCT with serious flaws or indirect evidence | Other alternatives may be equally reasonable |
| Weak recommendationeVery-low-quality evidence | Evidence for at least one of the critical outcomes from unsystematic clinical observations or very indirect evidence | Other alternatives may be equally reasonable |
RCT: randomized control trials; OS: observational studies.
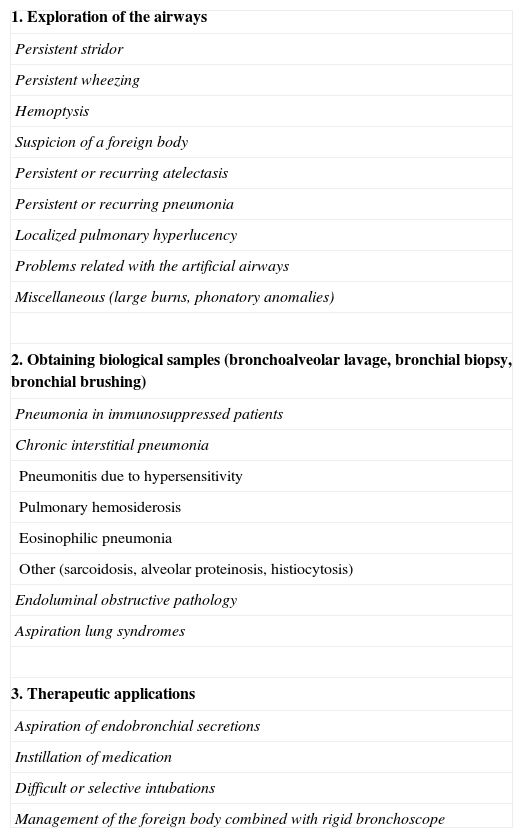
Flexible bronchoscopy (FB) enables us to obtain anatomical and dynamic information of the airways and to perform cytological and microbiological studies.2 Its indications arise with the need to respond to symptoms or radiological anomalies that cannot be explained by non-invasive methods3 or to obtain samples from the lower airways4 (Table 2). In children, compared with rigid bronchoscopy (RB), it is not a good therapeutic tool,5 although it does facilitate procedures that can resolve some of the problems in children's airways.6
Indications for Flexible Bronchoscopy in Pediatrics.
| 1. Exploration of the airways |
| Persistent stridor |
| Persistent wheezing |
| Hemoptysis |
| Suspicion of a foreign body |
| Persistent or recurring atelectasis |
| Persistent or recurring pneumonia |
| Localized pulmonary hyperlucency |
| Problems related with the artificial airways |
| Miscellaneous (large burns, phonatory anomalies) |
| 2. Obtaining biological samples (bronchoalveolar lavage, bronchial biopsy, bronchial brushing) |
| Pneumonia in immunosuppressed patients |
| Chronic interstitial pneumonia |
| Pneumonitis due to hypersensitivity |
| Pulmonary hemosiderosis |
| Eosinophilic pneumonia |
| Other (sarcoidosis, alveolar proteinosis, histiocytosis) |
| Endoluminal obstructive pathology |
| Aspiration lung syndromes |
| 3. Therapeutic applications |
| Aspiration of endobronchial secretions |
| Instillation of medication |
| Difficult or selective intubations |
| Management of the foreign body combined with rigid bronchoscope |
Its main cause is laryngomalacia,7 which tends to disappear within the first year and therefore does not usually require endoscopic revision, except in the cases of parental anxiety. In the cases of atypical presentation, biphasic character, prolonged persistence, suffocation crisis, eating difficulties, association with syndromes or malformations, history of intubation or diagnosis of severe laryngitis in children under the age of 6 months, a complete exploration is recommended8 as there may be anatomical, congenital or acquired anomalies9 (strong recommendation; high-quality evidence).
Suspicion of a Foreign BodyThe existence of respiratory symptoms and/or recurrent or persistent radiological images can be associated with the presence of an unnoticed foreign body. Under these circumstances, RB has a high rate of false negatives. In contrast, FB has a diagnostic exactitude of 100%. Therefore, if a diagnosis is mistaken, it is recommended to first perform FB10 (strong recommendation; high-quality evidence). Once extracted, FB can be very useful for confirming the absence of residues or of fragmented material, as occurs in the case of nuts.
Difficult-to-Control, Persistent WheezingFB is recommended in cases of difficult-to-control wheezing bronchitis, especially in small children, associated with asymmetries upon auscultation and/or radiological alterations11 as they could be due to a foreign body or structural anomalies of the tracheobronchial wall12 (strong recommendation; high-quality evidence).
HemoptysisHemoptysis is an uncommon and alarming symptom. In the absence of a known explanation (otorhinolaryngological infections or bronchiectasis), FB is required11 (strong recommendation; high-quality evidence). The most frequent causes are problems related with artificial airways, tracheostomies, primary alveolar hemorrhage, congenital vascular anomalies and infectious or inflammatory endobronchial pathologies, while tumors are the exception.13
Persistent/Recurring AtelectasisPersistence of more than 6 weeks, together with unexplained symptoms, makes FB recommendable14 (weak recommendation; high-quality evidence). The most frequent findings are mucus plugging, foreign bodies, extrinsic compression in cases of congenital cardiopathy,15,16 inflammatory granulation tissue or endobronchial tuberculosis.17,18
Persistent/Recurring PneumoniaMiddle lobe syndrome is a relatively frequent entity in pediatrics due to the anatomical characteristics of this bronchus. The observation of bacterial growth, a predominance of neutrophils in the bronchoalveolar lavage and evolution towards bronchiectasis in more than 50% of children with non-atopic wheezing19 has renewed interest in this syndrome.20 It has been proposed that the syndrome, if it cannot be resolved with treatment, indicates a study with FB21 (strong recommendation; high-quality evidence).
Localized HyperlucencyWhen its presence is not associated with congenital or post-infectious causes, localized hyperlucency requires carrying out FB as there may be air trapping due to a valvular mechanism, which is usually secondary to an intrinsic obstruction (foreign body, bronchomalacia,22 granulation tissue) or extrinsic compression due to adenopathies, vessels that are aberrant, anomalous and/or increased in size, or congenital or acquired mediastinal masses23 (strong recommendation; high-quality evidence).
Problems Related With Artificial Airways24FB is essential for the diagnosis of problems that arise during in/extubation and for monitoring and following patients with tracheostomy25 (strong recommendation; high-quality evidence). The most frequent findings are laryngeal edema, subglottic stenosis and tracheobronchial granulation tissue secondary to trauma caused by endotracheal tubes and cannulae or by repeated aspirations.26
Miscellaneous27Phonatory abnormalities: abnormal or persistent, aphonic crying can be due to the unilateral paralysis of a vocal cord generally associated with trauma during birth, hydrocephalus or post-operative cardiopathy, juvenile papillomatosis or aspiration during birth. The association with stridor warrants carrying out FB with a complete examination of the airway28 (strong recommendation; high-quality evidence). Other situations indicating FB include: persistent cough without diagnosis by non-invasive techniques (as it may be due to entities such as bacterial malacia or bronchitis.)29 and the evaluation of injury secondary to burns or inhalation of toxic substances.30
Bronchoalveolar Lavage and Biopsy SamplesFB is widely used in researching lung infiltrates that are unclarified, localized or diffuse, with interstitial, alveolar, miliary or nodular pattern, when diagnosis is not possible by means of other less-invasive methods (strong recommendation; high-quality evidence).
Therapeutic ProceduresAspiration of SecretionsFB can be useful for resolving atelectasis due to the retention of secretions or mucus plugging. The percentage of radiological re-expansion is variable, generally no higher than 50%. In cystic fibrosis31 (weak recommendation; low-quality evidence) or in plastic bronchitis (moderate recommendation; low-quality evidence) endoscopic lavage and aspiration can be indicated together with the instillation of mucolytics32 (MESNA or DNasa).33 In alveolar proteinosis, repeated BAL is the recommended treatment34 (strong recommendation; high-quality evidence).
Difficult and Selective IndicationsBronchoscopy can act as a guideline for intubation in cases of craniofacial anomalies and polymalformative syndromes,35 or in selective bronchial intubation (strong recommendation; high-quality evidence).
Extraction of Foreign BodiesAlthough there are publications that endorse the good performance of FB,36,37 it is usually a complicated procedure in small children. In these patients, RB is preferred as it offers the advantages of general anesthesia, assisted ventilation, larger instruments and a greater variety of accessories38 (strong recommendation; high-quality evidence). The ideal procedure would be to initiate with FB, which allows for greater reach in the exploration and the identification of the foreign body, extraction with RB, and a final revision with FB in order to rule out a residual foreign body.10 In some cases in which the clinical and/or radiological information is conclusive, the procedure may be directly initiated with RB.10
Other ProceduresFB can be of use in the peri-operative management of tracheoesophageal fistulas,39 both isolated or recurrent, as it allows for its identification and canalization, facilitating the surgical approach40 (weak recommendation, low-quality evidence). In other techniques such as bronchial permeabilization or the placement of prosthesis, the small size of the FB can be either a complication or an impediment, and therefore RB should be used41 (strong recommendation; high-quality evidence).
ContraindicationsThe indication for FB should be individualized, evaluating the risk/benefit for each patient. Some absolute contraindications impede performing bronchoscopy: severe refractory hypoxemia, hemodynamic instability, uncorrected hemorrhagic diathesis or the lack of authorization for the procedure by the parent or guardian. There are some relative contraindications determined by the experience of the team or the level of critical care of the hospital: very premature newborns, congenital cyanotic cardiopathies with an increase in bronchial collateral circulation, severe pulmonary hypertension or coagulation alterations.
ComplicationsPediatric FB is in general a safe procedure. The possible complications depend on the patient's risk factors, the type of procedure done, inadequate sedation/anesthesia, choice of instruments of an improper size and the inexperience of the bronchoscopist. The main complications are as follows.
Nasal Trauma and EpistaxisThese are the most frequent. Compression and/or topical adrenalin are sufficient for its control.
Desaturation and HypoxemiaThese may be due to causes inherent of the procedure and patient, or to the excess or deficit of sedation. FB increases the resistance of the airway which, together with sedation, can motivate hypoxemia and hypercapnia, which can persist 15–20min after the exploration. BAL and prolonged aspiration maneuvers, mainly in newborns and infants, favor alveolar collapse and increase its risk. Due to vagal irritation, or in children with bronchial hyperreactivity, there may also be some degree of bronchospasm. These changes can attenuated by maintaining a stable hemodynamic situation, choosing instruments of the proper size, previously administering bronchodilators and supplemental oxygen, optimizing the sedation-anesthesia and performing the procedure quickly.42
Cough and/or BronchospasmThese may be related with not enough sedation or the instillation of topical anesthesia.
Trauma and Obstruction of the AirwayAlso related with the choice of bronchoscope of improper size or forcing the way through a stenotic area, causing edema, hypersecretion and greater obstruction.
HemorrhageAspiration and brushing or biopsies usually cause small transitory hemorrhages facilitated by the existence of granulation tissue, tumors, bronchiectasis or hemorrhagic diathesis. Severe hemorrhage is exceptional, and can be associated with overly vascularized endobronchial lesions, which are rare in children, BAL in patients with coagulation disorders and especially transbronchial biopsy.
In general, hemorrhaging will let up spontaneously in a few minutes, but in cases of severe hemorrhage, lavage with cold physiological saline solution can be of use (5ml aliquots) or with adrenalin at 1/20 000–1ml of adrenalin at 1/1000, diluted in 20ml of physiological saline solution (strong recommendation; low-quality evidence). If the hemorrhage persists, it is recommended to place the patient in lateral decubitus, with the bleeding lung in a declining position, and to insert the bronchoscope in a segmental or subsegmental bronchus, aspirating continuously for 3–5min in order to produce a collapse of the distal bronchial walls (strong recommendation; low-quality evidence). On rare occasions, compression is needed with a 3 Fr Fogarty catheter or selective guided intubation. In these cases, RB could be necessary to explore the area of the bleeding and to aspirate the large clots. In one study, it has been suggested that the endobronchial instillation of pro-coagulation substances, such as tranexamic acid, can be effective, although the evidence for its recommendation is still limited43 (weak recommendation; low-quality evidence).
PneumothoraxPneumothorax is a rare complication although it can occur with transbronchial biopsy. In other cases, the misuse of the bronchoscope (uncontrolled pressure with the tip, abrupt movements, especially if there is coughing, or using either O2 or pressured air through the working channel) could cause them.44
Fever and InfectionsFever is frequent, appearing in up to 15% of the procedures, especially if BAL had been carried out. It is related with the release of cytokines or with transitory bacteriemia, mainly in immunosuppressed patients. More rarely, FB could give rise to infectious complications, both for the patient (dissemination to healthy areas of the lung or transmission of contaminated material from a previous exploration) as well as for the health-care professional (transmission of M. tuberculosis).
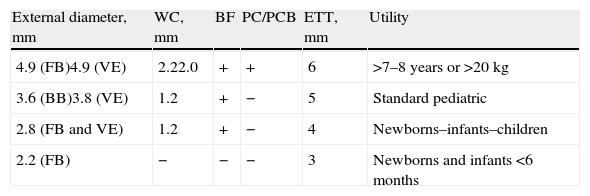
InstrumentsIn pediatrics, different models of bronchoscopes are used depending on the age of the child or the size of the endotracheal tube through which it is introduced (Table 3). The external diameter of the flexible tube and the working channel vary according to the type of bronchoscope used. There are four different bronchoscopes used in children, sized according to their external diameter: 2.2mm, 2.8mm, 3.5mm and 4.9mm.45 The smallest (2.2mm) does not have a working channel and is therefore only used for inspecting the airway.46 The 2.8mm and 3.5mm scopes have a 1.2mm channel. The 4.9mm scope has a 2.2mm channel, which enables protected brushing and biopsies and is more effective in aspirating secretions. This model can be used in children either over the age of 7 or weighing more than 20kg. Currently, bronchoscopy is being substituted with video-assisted bronchoscopy, which provides greater resolution and storage of the images in digital format.
Pediatric Flexible Bronchoscopes and Video-Assisted Endoscopes.
| External diameter, mm | WC, mm | BF | PC/PCB | ETT, mm | Utility |
| 4.9 (FB)4.9 (VE) | 2.22.0 | + | + | 6 | >7–8 years or >20kg |
| 3.6 (BB)3.8 (VE) | 1.2 | + | − | 5 | Standard pediatric |
| 2.8 (FB and VE) | 1.2 | + | − | 4 | Newborns–infants–children |
| 2.2 (FB) | − | − | − | 3 | Newborns and infants <6 months |
WC: diameter of the working channel; PC/PCB: protected catheters and protected catheter brushes; FB: flexible bronchoscope; BF: biopsy forceps; ETT: minimal internal diameters of endotracheal tubes for the passage of the bronchoscope; VE: video endoscopes.
- -
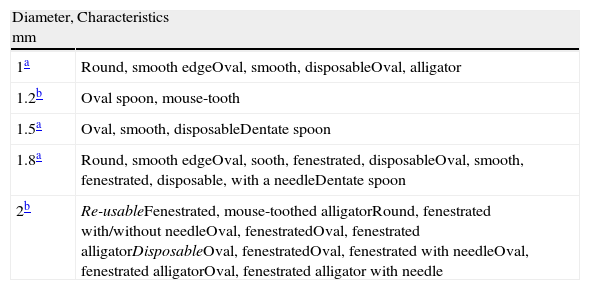
Biopsy forceps: the most often used are alligator forceps and they are used in bronchial as well as transbronchial biopsies. There are other models with smooth edges, with a central needle, fenestrated or not, revolving or swinging. All these are shown in Table 4.
Table 4.Biopsy Forceps Available for Pediatric Bronchoscopes.
Diameter, mm Characteristics 1a Round, smooth edgeOval, smooth, disposableOval, alligator 1.2b Oval spoon, mouse-tooth 1.5a Oval, smooth, disposableDentate spoon 1.8a Round, smooth edgeOval, sooth, fenestrated, disposableOval, smooth, fenestrated, disposable, with a needleDentate spoon 2b Re-usableFenestrated, mouse-toothed alligatorRound, fenestrated with/without needleOval, fenestratedOval, fenestrated alligatorDisposableOval, fenestratedOval, fenestrated with needleOval, fenestrated alligatorOval, fenestrated alligator with needle - -
Brushes: used for bronchial cytology studies and to test signs of inflammation, infection and malignancy.
- -
Transbronchial aspiration needles: used for diagnosing lung nodules or lesions near the bronchial tree. They are not frequently used in children.
- -
Accessories for extracting foreign bodies: although the standard norm is not to extract foreign bodies with FB, it is useful to have the equipment available in order to recover either small fragments or those from more distal areas that are not accessible with the rigid bronchoscope. There are 3 models: mini-forceps, forceps and extraction baskets (Table 5).
Table 5.Accessories for the Extraction of Foreign Bodies With Flexible Bronchoscope.
- -
Balloon catheter: used to plug hemorrhagic lesions.
- -
Endotracheal tube adapter: used in intubated patients to allow for simultaneous ventilation.
If possible, FB should be performed in patients under mild sedation, enough to tolerate the technique and the disorientation, permitting spontaneous breathing and thus reducing the risk of hypoxia. The previous conditions of the child will determine the type of access and the characteristics of the exploration.
Prior to carrying out the technique, the procedure will be explained, in comprehensible terms, to the child and/or parents and informed consent will be obtained. This will reduce the anxiety of family members and increase collaboration. A detailed clinical history should be available, including any circumstances that could affect the exploration (bronchial hyperresponsiveness, obstructive sleep apnea syndrome, allergies, cardiopathy and coagulation alterations).
The procedure is simple, well-tolerated and generally does not require hospitalization. The child should not ingest water during the 2h previous to the technique nor any food during 6h.
Options for Introducing the Flexible Bronchoscope- -
Nasal: this requires IV sedation and topical anesthesia of the nostrils (2% lidocaine).
- -
Nasal with a facial mask: provides O2 at 100% and continuous positive airway pressure support.
- -
Oral through a laryngeal mask: enables exploration of the lower airways from the glottic region.
- -
Endotracheal tube or tracheostomy: only allows for the observation of the lower airway.
- -
Rigid bronchoscope: its larger diameter accommodates the passage of the flexible bronchoscope.
The operator sits at the head of the patient, with the gurney in low position in order for the disposition of the equipment to avoid a curve. Forced curves in the scope can damage its fibers and make it difficult to handle. If the access is oral, a mouth prop or bite block is placed in the patient's mouth, which is not necessary if the access is through the nasopharynx. The bronchoscope is chosen to be proper for the child's age, and the distal end is lubricated. It is connected to the aspiration system, with pressure between 25 and 120cmH2O, interspersing appropriate receptors for sample collection.
If the access is nasal, the anatomy and the functionality of the pharyngeal and laryngeal structures (sublingual glands, tonsils, arytenoids, epiglottis and vocal cords) are studied. In cases where the larynx cannot be visualized (flaccidity, secretions), the line of air or oxygen can be connected to the working channel, at 1–2l/min, in order to exert positive pressure to clean or open the area. It is not advisable to conduct such a procedure placing the bronchoscope below the larynx given the risk for pneumothorax (weak recommendation; low-quality evidence).
The translaryngeal passage is done by centering the end of the bronchoscope in the angle of the anterior corner of the vocal cords, introducing it by means of posterior flexion when the patient inhales. In order to make the passage easier and to prevent the appearance of laryngeal spasm, a local dose of 1% lidocaine can be administered through the working channel. After reaching the subglottic space, new doses of lidocaine can be instilled in the lower airways, without ever surpassing the calculated maximal dose. After each instillation, a small volume of air should be introduced through the bronchoscope channel to completely empty it.
During the procedure, it is necessary to observe the axis of the trachea and its movement during breathing, the presence of cartilage, pars membranacea, carina, possible compression-displacement, pulse areas, etc. The exploration of the bronchial tree should be systematic, complete and sequential.47 In newborns weighing less than 1kg, the scope may be introduced into the main bronchi and the opening of the lobes may be observed. In newborns weighing more than 2.5kg, practically all the segments can be examined, except those of the upper lobes. At more than 6–8kg, there should be no problems to explore all the segments. The airway should be checked for possible anatomical anomalies, while observing the appearance of the mucosa (pale, erythematous, friable, thin or thick), the characteristics of the secretions (scant, moderate or abundant; localized or diffuse; mucous, purulent, hemorrhagic), and proceed with the taking of samples (secretions or tissues) necessary.
Post-Bronchoscopy CareThe patient should be placed in a comfortable position. The patient should be watched for the appearance of complications. Full consciousness needs to be recuperated and oral tolerance should be checked before making the decision to release the patient. No food or drink should be offered until the swallowing reflex reappears (2h after the procedure).
Bronchoalveolar LavageBAL allows for the evaluation of the cell and biochemical constituents of the epithelial surface of the lower respiratory tract or the presence of microorganisms, by means of the instillation and later aspiration of liquid in one or several lung segments.
Pre-requisitesChest radiographs and, if possible, computed tomography in order to outline the most ideal segment for BAL. In certain patients, platelet count higher than 60 000/mm3 and Quick index >50%.
TechniqueIf bacteriological studies are going to be ordered, BAL should be done first, before exploring the bronchial tree, avoiding aspirating secretions with the aim of impeding, or reducing the contamination of the suction channel by the oropharyngeal flora (strong recommendation; low-quality evidence). Likewise, lavage should be done before other techniques (biopsy, brushing or needle aspiration) that could cause hemorrhages and affect the results. Refrain from the instillation of topical anesthesia in the segment for lavage, as this could inhibit bacterial growth.
LocalizationIn children with localized lung lesions, BAL should be performed in the most affected segment or lobe. In diffuse disease, any place may be chosen, although the most appropriate segments are the middle lobe (ML) and the lingula, both due to their accessibility and easy impaction of the bronchoscope as well as the greater recovery of the liquid instilled as it is favored by the pull of gravity (strong recommendation; high-quality evidence). In infants, one of the most accessible areas is the right lower lobe.
Liquid Used, Methods of Instillation and RecuperationSterile isotonic saline is used, at room temperature or at 37°C to diminish the cough reflex.44 It is introduced through the bronchoscope channel with a syringe, followed by the instillation of sufficient air in order to completely empty it. Afterwards, the liquid that was introduced is aspirated by either manual or mechanical aspiration through a circuit made up of several interconnected recipients between the working channel and the aspiration, applying an intermittent negative pressure between −25 and −100mmH2O (strong recommendation; low-quality evidence). Excessive negative pressure should be avoided as it could lead to the collapse of the distal airway, impeding the recovery of the liquid.
Liquid VolumesChildren <20kg: a total of 3ml/kg, divided into 3 equal fractions; children ≥20kg: aliquots of 20ml, up to a total final volume of 3ml/kg (strong recommendation; low-quality evidence).
Processing of the Recovered LiquidThe volume instilled and the volume recovered both should be recorded. A recovery of 40% with few epithelial cells is accepted, although some authors48 admit ≥10%. In healthy children, between 43% and 85% of the volume introduced can be recovered, although the first sample is usually clearly less than the remainder. The first aliquot is considered the bronchial sample. It has more neutrophils and less lymphocytes than the remainder. For this reason, it should be separated from the rest for microbiological cultures, unless it is necessary to analyze specifically the cell component in bronchial inflammatory processes like asthma. The other aliquots offer comparable results, and therefore can be joined in one container. The processing of the liquid obtained should be done within 4h, and until processing it should be kept at 4°C to favor cell viability.
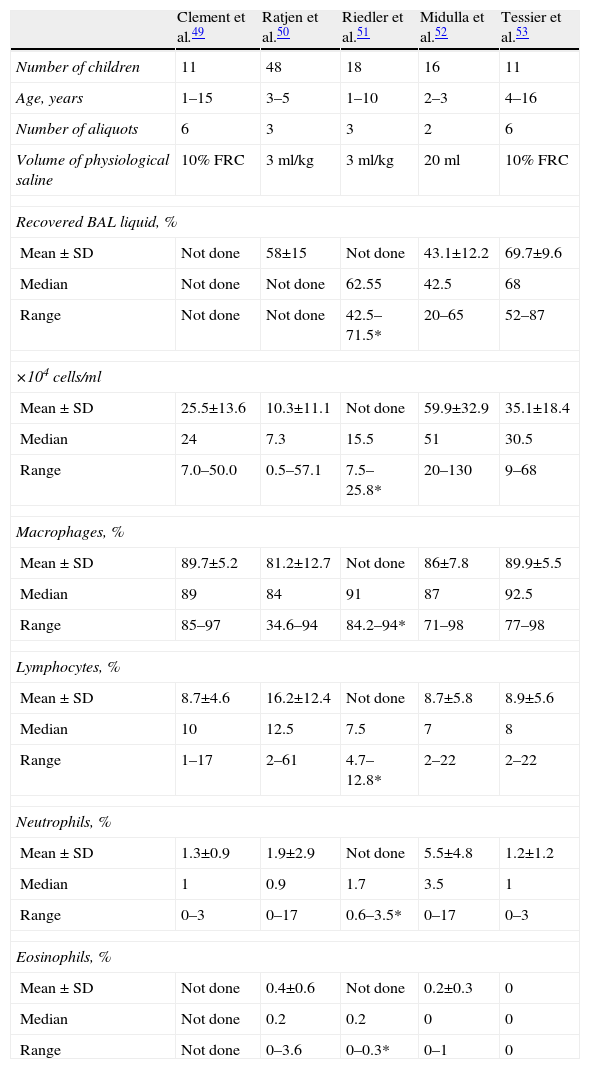
CellularityTotal cell count should be determined (considered valid with >300–500cells/ml) as should the differential formula. Studies on the normal values for children do not include many cases, many of which are not always absolutely healthy, and they have different methodologies (Table 6).49–53
Differential Cell Count in the Bronchoalveolar Lavage of Different Studies in Normal Children.
| Clement et al.49 | Ratjen et al.50 | Riedler et al.51 | Midulla et al.52 | Tessier et al.53 | |
| Number of children | 11 | 48 | 18 | 16 | 11 |
| Age, years | 1–15 | 3–5 | 1–10 | 2–3 | 4–16 |
| Number of aliquots | 6 | 3 | 3 | 2 | 6 |
| Volume of physiological saline | 10% FRC | 3ml/kg | 3ml/kg | 20ml | 10% FRC |
| Recovered BAL liquid, % | |||||
| Mean±SD | Not done | 58±15 | Not done | 43.1±12.2 | 69.7±9.6 |
| Median | Not done | Not done | 62.55 | 42.5 | 68 |
| Range | Not done | Not done | 42.5–71.5* | 20–65 | 52–87 |
| ×104cells/ml | |||||
| Mean±SD | 25.5±13.6 | 10.3±11.1 | Not done | 59.9±32.9 | 35.1±18.4 |
| Median | 24 | 7.3 | 15.5 | 51 | 30.5 |
| Range | 7.0–50.0 | 0.5–57.1 | 7.5–25.8* | 20–130 | 9–68 |
| Macrophages, % | |||||
| Mean±SD | 89.7±5.2 | 81.2±12.7 | Not done | 86±7.8 | 89.9±5.5 |
| Median | 89 | 84 | 91 | 87 | 92.5 |
| Range | 85–97 | 34.6–94 | 84.2–94* | 71–98 | 77–98 |
| Lymphocytes, % | |||||
| Mean±SD | 8.7±4.6 | 16.2±12.4 | Not done | 8.7±5.8 | 8.9±5.6 |
| Median | 10 | 12.5 | 7.5 | 7 | 8 |
| Range | 1–17 | 2–61 | 4.7–12.8* | 2–22 | 2–22 |
| Neutrophils, % | |||||
| Mean±SD | 1.3±0.9 | 1.9±2.9 | Not done | 5.5±4.8 | 1.2±1.2 |
| Median | 1 | 0.9 | 1.7 | 3.5 | 1 |
| Range | 0–3 | 0–17 | 0.6–3.5* | 0–17 | 0–3 |
| Eosinophils, % | |||||
| Mean±SD | Not done | 0.4±0.6 | Not done | 0.2±0.3 | 0 |
| Median | Not done | 0.2 | 0.2 | 0 | 0 |
| Range | Not done | 0–3.6 | 0–0.3* | 0–1 | 0 |
FRC: functional residual capacity; SD: standard deviation; BAL: bronchoalveolar lavage.
For the diagnosis of bacterial infections, Gram stain, quantitative colony-culture and, in some cases, intracellular bacteria studies should be done. The detection of more than 1% of epithelial squamous cells can indicate an excessive contamination of the sample by oropharyngeal flora. Isolations ≥10 000UFC/ml are considered significant (strong recommendation; high-quality evidence). The detection of microorganisms that do not form part of the usual oropharyngeal flora (M. tuberculosis, Legionella, Nocardia) is always pathological (strong recommendation; high-quality evidence).
Diagnostic Indications- -
Lung infections in immunocompromised children, those with transplantation or oncological diseases, who initiate with acute dyspnea and hypoxemia with new diffuse interstitial infiltrates on chest radiograph: in this case, the technique should be done before initiating antibiotic therapy or if there is no improvement after 48h of treatment. The diagnostic performance is high at around 80%.
- -
Immunocompetent children with severe pneumonia who do not respond properly to treatment or who require mechanical ventilation, and in nosocomial pneumonia in intubated and ventilated children.
- -
Non-infectious lung diseases: this is the diagnostic method of choice for alveolar proteinosis, alveolar hemorrhage, pulmonary histiocytosis and pneumonia due to lipid accumulation. In the first, the milky appearance of BAL is characteristic, as are the presence of lipoprotein-like PAS (+) extracellular material, alcian blue (−) and the detection of the typical lamellar bodies by electron microscope. In the alveolar hemorrhage, the reddish appearance of the BAL, which increases in successive aliquots, induces suspicion. Perls stain identifies the hemosiderophages, although they may be absent in recent (less than 48h) or remote (more than 12 days) hemorrhages. In adults, the stain is considered positive when there is more than 25% of macrophage stained, but in children a slight increase can already be significant. The identification of Langerhans cells induces suspicion of histiocytosis X. For its confirmation, the presence of ≥5% of CD1a (+) cells or the positivity for S-100 protein is required.
- -
Acute eosinophilic pneumonia: eosinophils in the lavage >25%.
- -
Pneumonitis due to hypersensitivity: there is lymphocytosis, as in adults, but with a normal CD4/CD8 relationship. It may be useful to evaluate the natural killer cells and the expression of antigen–DR in the human leukocytes.
- -
Aspiration syndromes: these can run their course with a high lipid index (percentage of macrophages loaded with lipids), although the threshold for positivity and its significance is up for debate.
- -
Interstitial lung disease: can provide information on the cell pattern and direct the diagnosis.
- -
Infants and toddlers with severe recurrent wheezing: the type of cells detected or certain inflammatory components (cysteinyl-leukotrienes) could lean towards a phenotype, asthmatic or not. At these ages, bronchial inflammation is usually basically neutrophilic, the same as in the severe bronchiolitis or bacterial bronchitis of children with chronic cough or recurring respiratory pathology.
- -
Cystic fibrosis: BAL in the first stages of the disease can reveal the existence of inflammation and/or bronchial infection.31
One accepted application is the treatment of alveolar proteinosis. In children under the age of 10–12, unlike in older children or in adults, it is not possible to use a double lumen endotracheal tube and ventilate one lung while another is washed. The standard technique involves placing, with the help of a flexible bronchoscope, a balloon catheter or a small endotracheal tube in one bronchus, inflate the balloon to isolate the bronchial tree and carry out a massive BAL through the tube or catheter, while ventilating the other lung by means of a laryngeal mask or an oropharyngeal tube (strong recommendation; low-quality evidence).
Bronchial BiopsyThis is done in an endobronchial lesion or in the mucosa. In this case, it allows for the epithelium, the basal membrane and, occasionally, the smooth muscle to be studied.54 It can be used for the diagnosis of granulomatous diseases (tuberculosis or sarcoidosis), endobronchial tumors or chronic fungal diseases.55 It is also appropriate for obtaining ciliated cells in the diagnosis of primary ciliary dyskinesia,56 although nasal brushing, with equal performance and greater simplicity, is the most recommendable technique57,58 (strong recommendation; high-quality evidence); it is used in research studies in children with cystic fibrosis,59,60 asthma61–63 or recurrent wheezing bronchitis,64,65 and can be useful for the management of difficult-to-control asthma.66
To carry out the procedure, standard 1.8mm diameter forceps are used, utilizing the 4.9mm bronchoscope with a 2–2.2mm channel. The use of a laryngeal mask for the sedation and anesthesia allows for the use of this bronchoscope for biopsies in children over the ages of 2–2.5.67 With the smaller 1.1mm forceps, the sample is smaller, but some groups have achieved representative samples.68 It is recommended to obtain 3–5 samples in the case of endobronchial lesions, and a minimum of 3, in the biopsies of the mucosa that are taken from a subsegmental bronchial carina, between the third and the fourth generation. The use of deep sedation or general anesthesia avoids patient movement during the procedure, increasing safety.69
The technique is well tolerated and no important bleeding or pneumothorax has been reported. In biopsies of endobronchial lesions, the risk of bleeding increases, and if these are very vascularized, it is advisable to previously instill 1–2ml of adrenalin at 1/20 000 and to carefully evaluate the risk–benefit relationship beforehand. Routine chest radiography is not necessary afterwards (strong recommendation; low-quality evidence).
Transbronchial BiopsyWith this technique, pulmonary parenchyma can be obtained for microscopic analysis, avoiding the need for thoracotomy. The majority of these procedures are carried out in lung transplant patients,70 although it can also help diagnose other pediatric lung diseases.
This is the technique of choice for the diagnosis of rejection episodes in children with lung transplantation, with a sensitivity of 72%–94% and specificity of 90%–100% (strong recommendation; high-quality evidence). It has less value for the diagnosis of chronic rejection or bronchiolitis obliterans as in these cases the distribution of the lesions is very patchy and bronchioles are not always observed in the sample obtained. It should always be done when there is a suspicion for acute rejection, and selectively at 7–15 days, 6 weeks, 3, 6, and 12 months post-trasplant.71 Some workgroups only indicate the procedure after 6 months in cases of suspected rejection.
It is not recommended for the diagnosis of idiopathic interstitial pneumopathies in children, in which case open biopsy is indicated72 (strong recommendation; high-quality evidence). It can be useful in granulomatous diseases, such as miliary tuberculosis or sarcoidosis, and also in extrinsic allergic alveolitis, eosinophilic pneumonia, vasculitis, alveolar proteinosis, histiocytosis X, alveolar microlithiasis, lymphocytic interstitial pneumonia and graft versus host disease.
In immunosuppressed patients,73 transbronchial biopsy could help establish the definitive diagnosis in infections due to cytomegalovirus, by demonstrating the presence of intranuclear inclusions or a positive immunohistochemical stain in the lung tissue, although given the focal nature of the infection, the frequency of positive results is only 11%–55%.
Before starting the test, it is necessary to have chest radiography, hemogram with platelets, coagulation tests, urea, creatinine and serum electrolytes. The procedure should be done with the patient under general anesthesia and either intubated or with a laryngeal mask, which gives better handling of the airway if the patient bleeds and makes for easier repetitive insertion and withdrawal of the bronchoscope. Afterwards, a follow-up radiograph is recommended within 2–4h, in order to rule out the production of a pneumothorax and maintain the child in observation until the day after the procedure.
The use of fluoroscopy makes the technique easier, providing visualization and localization of the forceps, including their distance from the pleura, for the puncture74 (strong recommendation; moderate-quality evidence).
With the 4.9mm bronchoscope or with the rigid bronchoscope, standard 1.8mm diameter adult biopsy forceps can be used. For the 3.6mm or 2.8mm bronchoscopes, 1.1mm diameter smooth-edged biopsy forceps have been developed. These are used less often as the quantity of tissue that they extract is less and not as proper or standardized for histological studies. Nevertheless, it is possible to obtain representative samples in 85% of the procedures. Some authors prefer using the rigid bronchoscope for biopsies in small children as they accommodate the larger-sized standard forceps. However, it is more difficult to position the forceps in the segment desired and to access the upper lobes. The risk for pneumothorax is also greater.
If the affectation is focal, the biopsy is done in the altered lung lobe or segment. If the affectation is diffuse, it is usually done in the right lower lobe, which is the easiest and safest location. The middle lobe and lingula should be avoided due to the higher risk for pneumothorax. Biopsies will always be done in only one lung at each session, in order to avoid causing bilateral pneumothorax. It is reasonable to obtain 4–6 samples for the histological study of different segments of the biopsied lobe and one more sample if microbiological studies are necessary.
The most important complications are pneumothorax and hemorrhage. The incidence of pneumothorax oscillates between <1% and 3.5%26 and it is more frequent in cases of bullous pulmonary lesions, peripheral localization of the lesions, mechanical ventilation and in immunosuppressed patients.75 There is always a minimal amount of bleeding, which is self-limiting. Moderate (>25ml) and severe (>100ml) bleeding has been reported in 0.6%–5.4% of patients and, in some cases, death (1–2/1000 procedures).73 It is important to properly select the patients and not perform biopsy on those with risk for bleeding: immunosuppression, uremia, lymphoma, leukemia, renal transplant and coagulation disorders.
Bronchial BrushingThis may be used to study the bronchial mucosa or to culture bronchial secretions. It is a useful technique for the diagnosis of ciliary dyskinesia, tuberculosis, pneumonia in patients with mechanical ventilation and lung infections in immunosuppressed patients.76,77
Ideally, a protected brush should be used. It is made up of a brush housed in the interior of a catheter that, at the same time, is protected by another outer catheter. It is occluded in the distal end by a plug made up of an absorbable substance in order to prevent contamination on its way through the airway. The catheter is inserted through the channel of the bronchoscope until it surpasses the distal end by 3cm in order to avoid collecting secretions accumulated in the tip of the catheter. The device is pushed to dislodge the polyethylene glycol plug situated in at the distal end. The brush is moved outside the catheter and either the bronchial wall is brushed or the brush is rotated in the purulent secretions, if any are visualized, to take a sample. The brush is then retracted into the internal catheter, and then the internal catheter into the external catheter, and the bronchoscope is withdrawn.
The protected brush can only be used with the 4.9mm bronchoscope. With the 3.6 or 2.8mm bronchoscope, the non-protected brushes are used. In this case, upon withdrawing the brush through the working channel, the cells or the secretions may be lost. This risk diminishes if the brush is allowed to protrude from the bronchoscope (being careful not to touch the upper airway) or if the brush is hidden within the tip of the bronchoscope as it is being removed.
Once the brush is extracted, if it is protected, the outside is cleaned with 70% alcohol. If it is sent for culture, the brush is cut off with sterile scissors and it is deposited in a tube with 1ml sterile physiological saline solution. If cytological or histological studies are ordered, the brush may be shaken in the appropriate medium to release the cells, spread on a slide or the tip cut and deposited in 10% formaldehyde.
Possible complications of bronchial brushing are bleeding (especially in patients with coagulation alterations or renal problems) and pneumothorax.
Rigid BronchoscopyIndicationsFB has slowly been taking on a predominant role in pediatric bronchoscopy and, although it has substituted RB in most centers, rigid bronchoscopy is still widely used. The basic reason is the high incidence of foreign bodies in children. On the other hand, despite there being new ultrafine scopes for exploring newborns and infants, RB is still useful as a diagnostic or therapeutic tool when there is compromised ventilation, when extensive biopsies are necessary or when atelectasis should be resolved with the elimination of mucus plugging. The majority of the indications for RB are therapeutic78: endoscopic treatment of localized airway obstruction, extraction of foreign bodies, management of massive hemoptysis and therapeutic instrumentation of the airway (strong recommendation; high-quality evidence). Pediatric RB is a procedure that is not regularly performed in most centers, therefore learning this technique usually requires specific training, generally postgraduate and in a reference center.
Obstruction of the Central AirwaysThe larynx, trachea and main bronchi are the bane of various surgical pathologies that cause stenosis of the lumen, from tumor to inflammatory lesions. RB is the endoscopic procedure of choice when open surgery cannot be contemplated to treat these processes either with the application of laser, implantation of endoprosthesis or other therapies. Although some of these techniques, such as the application of laser, balloon dilation or the placement of tracheobronchial prosthesis (except those made of silicone) can be done by bronchoscope or even by radioscopic control, there is extensive consensus that the safest method and that which gives the best results is rigid bronchoscopy.
In critical stenosis of the common airway with a reduction of ≤20% of the predicted lumen and the patient's life at risk due to asphyxia, RB can be a life-saving procedure as it allows for the immediate restitution of the airway. It can dilate or perforate the nucleus of the tumor, opening the airway or progressively dilating the inflammatory stenosis. This not only avoids the need for tracheotomy if the obstruction is laryngeal, but it also the only option when the obstruction is located under the cervical trachea.
Extraction of Foreign BodiesRigid bronchoscopy is the procedure of choice for the safe and quick extraction of foreign bodies in children (strong recommendation; high-quality evidence). Nevertheless, in older children without compromised vital functions, FB, although it is more laborious and less effective (61% of successes compared with 97% for RB), can be an alternative.
Massive HemoptysisThe majority of hemoptysis can be controlled with conservative measures, bronchoscopy and embolization by arteriography, but when the hemoptysis is massive and it compromises vital functions due to flooding of the airway with blood, RB is necessary for aspiration and for extracting the clots through its large working channel. Rigid bronchoscopy also accommodates two essential maneuvers at the same time: it guarantees ventilation while achieving hemostasis of the bleeding point. The former is done by protecting the contralateral lung from flooding by means of effective aspiration and selective intubation. Once the ventilation is guaranteed, the following objective is to achieve hemostasis. For this, either electrocoagulation or laser can be used. The hemorrhage can also be controlled by plugging the bleeding point with the bronchoscope itself or with an endobronchial occlusion balloon.
Therapeutic Instrumentation of the AirwayAmong the therapies that are applied preferentially with RB are: laser, endoprosthesis, electrocoagulation, cryotherapy, argon plasma coagulation and balloon dilation. It can also be used as a guide in percutaneous tracheotomy and for the treatment of different lesions, such as tracheoesophageal fistulas or tracheobronchomalacia.
Instruments and EquipmentTypes of Rigid BronchoscopesPediatric bronchoscopes are shorter than those used in adults. They measure between 30 and 16cm long with an internal diameter between 3.2mm and 7mm. To these measurements, one must also add the thickness of the wall of the tube, which ranges between 2 and 3mm depending on the manufacturer, to obtain the external diameter. The beveled tip facilitates vision during intubation, and makes for easier passage through the vocal cords and stenosis and for mechanical resection. On its distal third, there are lateral holes, introduced by Killian, that ventilate the contralateral lung.
Some bronchoscope models have different ports of entry on their proximal end designed for different uses. Those of standard size are for connecting respirators and jet ventilation. Others allow for the simultaneous introduction of different types of fiber lasers and aspiration catheters. There are also optics of different thickness that adapt to the working channel.
In addition to the basic apparatus, it is convenient to have a video camera adaptable to the head of the optics. The variety of endoscopic forceps is extensive, but the main ones are: alligator mouth forceps for foreign bodies, universal forceps, spoon-shaped biopsy forceps and grasping forceps for soft foreign bodies.
TechniqueThe patient is positioned in supine decubitus with the bronchoscopist at his/her head. The ideal position for the visualization of the larynx and intubation is the combination of anterior flexion of the neck with hyperextension of the atlanto-occipital joint (flexion–extension or Boyde-Jackson position).
For intubation with the rigid bronchoscope, a laryngoscope can be used, using the standard tracheal intubation technique or directly using the RB; in this case, one may either look directly through the channel of the bronchoscope or use the straight optical viewfinder, which gives a better-quality magnified vision. The bronchoscope is held with the right hand and with the left the mouth is opened, protecting the incisors as well as the soft parts, lips and tongue from injury. The visualization of the arytenoids, with the vocal cords in front and the entrance of the esophagus behind, serves as a reference. In order to pass through the vocal cords, the bronchoscope is rotated on its axis clockwise until its tip coincides with the major axis of the rima glottidis. Once past the separated vocal cords, the rotation is undone. In the previously intubated patient, the point of the bronchoscope slips down on top of the orotracheal tube, using the tube as a guide until the tip comes to the point above the vocal cords; the orotracheal tube is then withdrawn and the RB pushed forward. Lastly, when there is a tracheotomy, the bronchoscope can also be introduced, carrying out what is known as a lower bronchoscopy in order to distinguish it from a translaryngeal or upper bronchoscopy. Once past the glottis, the ventilation system is connected to the lateral connections.
ComplicationsThe complications of RB are due to the instrumentation with the bronchoscope itself, the medication used, the ventilation technique, the underlying pathology, the experience of the endoscopist and the type of intervention (extraction of foreign bodies, laser, placement of endoprosthesis, etc.).
The introduction of the bronchoscope can injure teeth and the soft parts of the mouth, the lips and tongue, as they can become caught between the metallic tube and the teeth. During the maneuver, the bronchoscopist must avoid using the incisors as a leverage point in order to elevate the soft tissue and expose the glottis. To prevent this type of injury, a dental protector is used. When the bronchoscope goes through the glottis, there may be damage to the arytenoids or vocal cords. When it enters into the trachea, there may be a cardiovascular response that, in adults, is usually sympathetic-tonic with an increase in the heart rate and blood pressure, but in younger people and in children the response may be vagotonic with bradycardia.
The most frequent complications are related with anesthesia and ventilation. These are hypoxemia, hypercapnia, cardiac arrhythmias and barotrauma.
Conflict of InterestThe authors declare having no conflict of interests.
Please cite this article as: Pérez-Frías J, et al. Normativa de broncoscopia pediátrica. Arch Bronconeumol. 2011;47:350–60.