The current SARS-CoV-2 pandemic offers an opportunity to reflect on the use of oxygen therapy in the management of acute respiratory failure that often accompanies Covid-19. At a time when scientific evidence filters all clinical practice, it is easy to see that oxygen therapy is often implemented in patients with acute respiratory failure with no real scientific basis1.
Retrospective studies already show that excessive oxygen use does not improve the prognosis of critically ill patients, and that hyperoxia is associated with higher mortality2,3. In the 1970s, Douglas et al.4 found that high oxygen levels (FiO2 > 0.7) increased pulmonary venous admixture due to the appearance of absorption atelectasis and the suppression of the hypoxic pulmonary vasoconstriction reflex. Other authors have shown that lower FiO2 levels (even only 0.3) can cause the same alterations in patients with pre-existing lung disease5.
Excessive oxygen intake can impair gas exchange by increasing oxidative stress and inflammation (the Lorrain-Smith effect). In the current epidemiological context, oxidative stress has been placed in the spotlight by the recent description of the hypercytokinemia associated with SARS-CoV-2 pneumonia6. Excessive doses of oxygen increase the production of reactive nitrogen and oxygen species (RNS, ROS), the recruitment and activation of inflammatory cells, and the release of pro-inflammatory mediators (IL-1α, IL-6, IL-8, or TNF-α), with local (lung damage and infection and sepsis) and distant (tissue damage and multiorgan failure) consequences. Although these effects are magnified when high FiO2 levels are maintained for prolonged periods (>12–24 h), shorter periods of hyperoxia are also associated with increased morbidity and mortality7. Hyperoxia, then, could be an indirect marker of poor prognosis in severe patients3.
Paradoxically, hyperoxia can reduce systemic oxygen consumption, probably due to the redistribution of blood flow to protect vital organs from the non-physiological effects of high FiO2 (functional shunt). Higher than normal levels of PaO2 also trigger arteriolar vasoconstriction by local mechanisms, a phenomenon that could explain the worse progress of some patients with myocardial infarction, stroke, head injury, cardiac arrest, or sepsis. Tissue hyperoxia, furthermore, promotes lesion development in ischemia-reperfusion situations8.
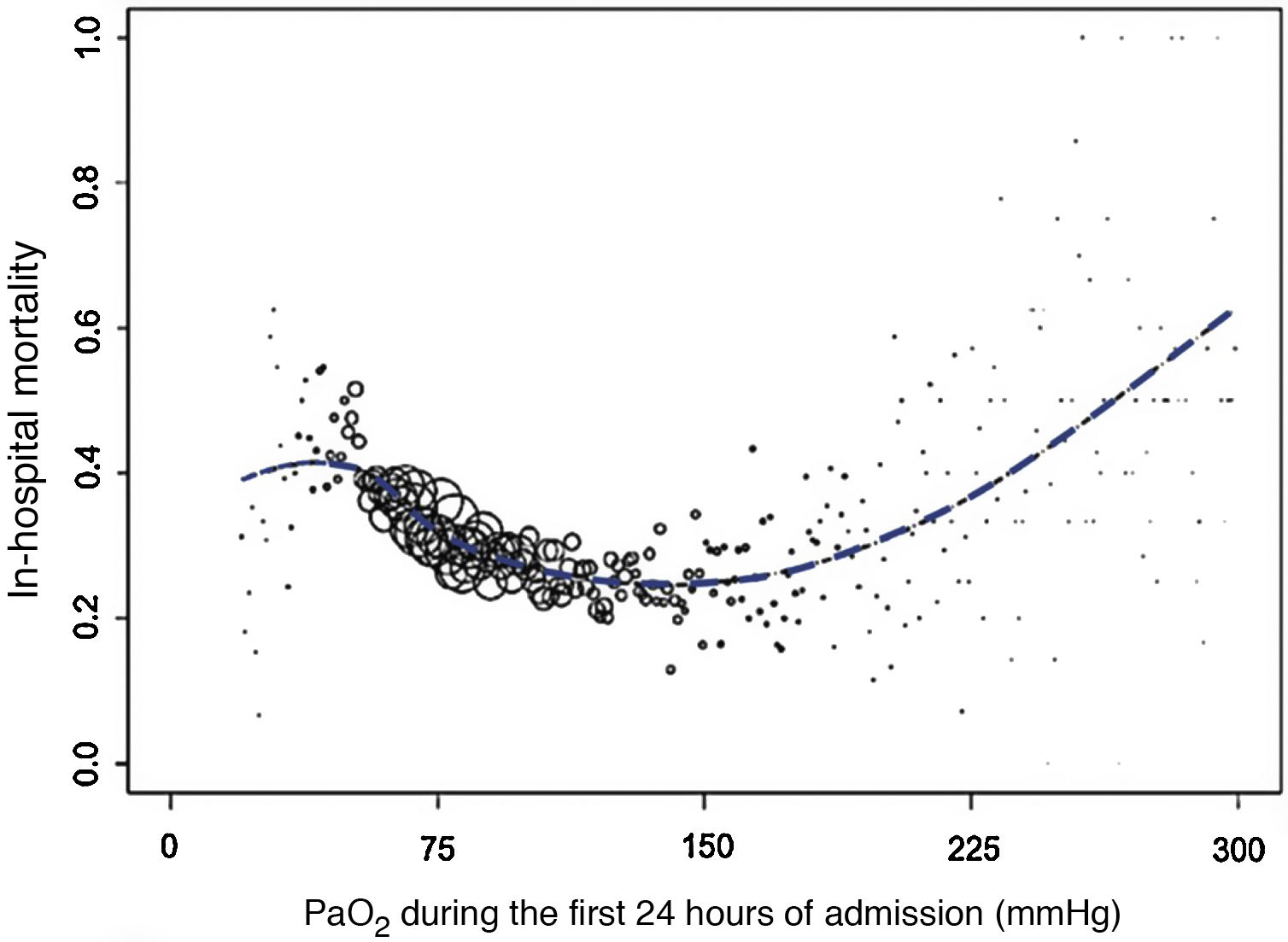
In short, hyperoxia can be as dangerous as hypoxemia. A multicenter retrospective study of 36,307 patients hospitalized in intensive care units (ICU) showed that PaO2 during the first 24 hours of admission was an independent predictor of hospital mortality2. PaO2-related mortality formed an asymmetric U with a clear plateau at the end of the lowest PaO2 values, linear increase on the hyperoxia side, and lowest mortality rates at a PaO2 value between 110 and 150 mmHg (Fig. 1).
In-hospital mortality values, on average, according to PaO2 (mmHg). The size of the circles represents the number of patients with the same PaO2 value. Adapted from De Jonge et al.2 with permission.
When expressed as a standardized mortality ratio2, hyperoxia was a predictor of mortality at PaO2 values >125 mmHg.
A recent clinical trial in patients with septic shock had to be discontinued prematurely after evidence emerged of excess in-hospital mortality in hyperoxic patients9. Hyperoxia also appears to be of little benefit during recovery after cardiac arrest. Helmerhorst et al.3 showed in an observational study that in-hospital mortality after cardiorespiratory arrest was the same as in patients with normal oxygen levels (PaO2 55–80 mmHg).
Hyperoxia does not benefit patients with severe acute respiratory failure. The effect of exposure to excess FiO2 (FiO2 > 0.5 when PaO2 was >80 mmHg) was analyzed in a study in 2994 patients with acute respiratory distress syndrome (ARDS). This study found that the higher the cumulative exposure at 5 days, the higher the mortality, the number of days with mechanical ventilation, and the length of hospital stay. Mortality also affected patients with mild, moderate, or severe ARDS, which meant that the relationship observed between mortality and cumulative exposure to high FiO2 was independent of disease severity10. A recent multicenter randomized trial in patients with ARDS confirmed that controlled oxygen therapy (PaO2 55–70 mmHg or SpO2 88%–92%) did not increase mortality at 28 days11.
Evidence in patients with acute chronic respiratory failure also supports controlling SpO2 values. Plant et al.12 showed that excessive use of oxygen therapy in patients with chronic obstructive pulmonary disease exacerbations was associated with respiratory acidosis and a greater number of ICU admissions. The authors pointed out that therapy should focus on maintaining PaO2 and SaO2 at 55–75 mmHg and SaO2 85–92%, respectively. A subsequent controlled trial showed higher mortality among patients receiving oxygen therapy not adjusted to achieve SaO2 values of 88%–92%13.
Acute respiratory care should, therefore, focus on improving lung function with the proper use of oxygen therapy, an approach that differs considerably from standard practice. Unnecessarily high FiO2 levels reduce the window of opportunity for switching treatment or adjusting FiO2 levels in the case of worsening respiratory status6. In acutely hypoxic patients, an SpO2 of 90%–94% seems to be a reasonable range. It accommodates normal fluctuations and is safe enough to prevent damage. High-flow systems (e.g., Venturi masks) that can be adjusted to achieve stable FiO2 are recommended for oxygen administration. The idea of setting a minimum PaO2 value to avoid tissue hypoxia should be discarded, and emphasis should be placed on setting a maximum safety value to avoid systemic toxicity (PaO2 <120 mmHg). In patients with hypercapnic respiratory failure, the SpO2 ranges should be lower (88%–92%) and ventilatory support (preferably non-invasive mechanical ventilation) should be considered if greater oxygen transport is needed (e.g., associated failure of other organs) or if hypercapnia persists despite controlled FiO2 (0.24–0.4). High-flow nasal cannula may be an alternative or a supplement to non-invasive mechanical ventilation in more severe patients14. In the specific case of patients with chronic obstructive pulmonary disease exacerbations, some guidelines recommend a maximum FiO2 of 0.28, a practice that is also advisable in the prehospital setting15. In the absence of hypoxemia, oxygen therapy should be avoided in patients with acute myocardial infarction, stroke, head injury, after cardiorespiratory arrest, and in patients with sepsis. In patients admitted to the ICU, restrictive oxygen therapy protocols (aimed at maintaining PaO2 within the physiological range) are well tolerated and may improve prognosis9.
In conclusion, a more liberal oxygen prescription is at odds with modern requirements for the development and approval of a treatment of any type. The use of automated oxygen flow titration devices could improve morbidity and mortality and reduce care costs, although they are unlikely to replace clinical monitoring16. In the meantime, we must stress that oxygen is a drug that must be prescribed for defined indications where the benefits outweigh the risks, and that the dose, the delivery system, the duration of administration, and response monitoring must be clearly specified17.
FundingThe authors declare that they have not received funding for this study.
Conflict of interestsThe authors state that they have no conflict of interests.
Please cite this article as: Valencia Gallardo JM, Solé Violán J, Rodríguez de Castro F. Oxigenoterapia. Consideraciones sobre su uso en el enfermo agudo. Arch Bronconeumol. 2022;58:102–3.