Patients with activating somatic mutations in the Epidermal Growth Factor Receptor (EGFR) have better clinical outcomes when treated with Tyrosine Kinase Inhibitors (TKI) over chemotherapy. However, the impact of the use of TKIs on overall survival outside clinical trials is not well established.
ObjectiveTo characterize and analyze the overall survival of a Caucasian population with NSCLC and EGFR mutations.
MethodsA retrospective cohort analysis of patients with NSCLC screened for EGFR mutations (exons 18–21) between October 2009 and July 2013 was conducted. Clinical and pathological characteristics, mutational EGFR status, treatment and overall survival were evaluated.
ResultsFrom the 285 patients which performed screening for EGFR mutations, 54 (18.9%) had mutations, 25 (46.3%) of which in exon 19 and 20 of which (37.0%) in exon 21. The occurrence of mutations was associated with female sex and non-smoking habits (both, P<.001). The median survival of the global population was 12.0 months, with a better overall survival in mutated than non-mutated patients (20.0 vs 11.0 months, respectively; P=.007).
ConclusionThese data contribute for a better knowledge of our lung cancer population concerning the mutational status and clinical outcomes, confirming a better overall survival for the patients with EGFR TKI sensible mutations.
Los pacientes con mutaciones somáticas activantes en el receptor del factor de crecimiento epidérmico (EGFR) obtienen mejor resultado clínico cuando se tratan con inhibidores de la tirosina cinasa (TQ) frente a quimioterapia. Sin embargo, el impacto de la terapia en inhibidores de TQ en la supervivencia global de los pacientes no está del todo establecido en la práctica clínica habitual.
ObjetivoCaracterizar y analizar la supervivencia global de una población caucásica con cáncer de pulmón no microcítico y mutaciones en el gen EGFR.
MétodosSe realizó un análisis retrospectivo de una cohorte de pacientes con cáncer de pulmón no microcítico con mutaciones en el gen EGFR (exones 18-21) entre octubre de 2009 y julio de 2013. Se evaluaron las características clínicas y patológicas, el estatus mutacional del gen EGFR, el tratamiento y la supervivencia global.
ResultadosDe los 285 pacientes que se cribaron para caracterización de mutaciones en el gen EGFR, 54 (18,9%) presentaron mutaciones, de los cuales 25 (46,3%) tenían mutaciones en el exón 19 y 20 (37,0%) en el exón 21. Se observó que la ocurrencia de mutaciones estaba asociada al género femenino y al no consumo de tabaco (p<0,001 en ambos casos). La supervivencia media de la población global fue de 12 meses, con una mejor supervivencia global en pacientes que presentaron mutaciones que en los que no las presentaron (20 vs. 11 meses, respectivamente, p=0,007).
ConclusiónEstos datos contribuyen a mejorar el conocimiento de nuestra población con cáncer de pulmón con relación a su estatus mutacional y el resultado clínico, confirmando una mayor tasa de supervivencia global en los pacientes con mutaciones en el gen EGFR sensibles a inhibidores de TQ.
Lung Cancer is the most frequent cause of cancer death.1 NSCLC is more often diagnosed in advanced stages,2 which reduces the therapeutic options to cytotoxic chemotherapy, with modest outcomes.3 Its poor prognosis turns this disease into an emergent area of investigation.
The Epidermal Growth Factor Receptor (EGFR) belongs to the ErbB family, composed by several transmembrane tyrosine kinase receptors.4 These receptors mediate extracellular growth factors such as the epidermic growth factor.4 Dysregulation of these receptors leads to uncontrolled proliferation and increased resistance to apoptosis5 as well as modified cell adhesion and increased migration capacity, facilitating neoplastic invasion and metastization.6
The EGFR tyrosine kinase domain is found in the exons 18–24 and the more relevant mutations are located in the exons 18–21.7 The most frequent EGFR mutations consist in in-frame deletions in exon 19, by the modification of the LREA amino-acid motif (delE746–750) and in missense mutations in exon 21 (L858R codon).8
The presence of EGFR mutations in NSCLC is associated to certain clinical characteristics as female sex, Asian ancestry, absence of smoking habits and histology of adenocarcinoma.8
EGFR tyrosine kinase inhibitors (TKI) were first used in a non-selective way in NSCLC treatment resulting in disappointing outcomes, being effective only in a small proportion of patients.9
The first evidence that EGFR mutations could be used as a target therapy emerged thirteen years ago.8 Since then, several clinical trials concluded that TKI were more effective than chemotherapy in the treatment of NSCLC patients with EGFR mutation,8,10 except when the EGFR mutation is in exon 20 (insertion mutations and T790m).11 Current recommendations support EGFR TKI as the first-line treatment in patients with advanced NSCLC and EGFR TKI sensible mutations.12,13
The knowledge of EGFR mutations and its relation with treatment outcomes was one of the most recent important steps in lung cancer management. Tumor-free progression and quality of life parameters are better with TKI when compared to chemotherapy,14,15 but until now, only studies with Afatinib proved survival advantage for the exon 19 deletion over chemotherapy.16 Real life data, outside clinical trials information, is poor. In our population neither the correlation between specific clinical features and EGFR mutations nor the effect on survival are well established.
In the present study, a cohort of NSCLC patients tested for EGFR mutation was characterized and a survival analysis was conducted.
Patients and MethodsStudy Design and Population SelectionRetrospective study of a cohort of lung cancer patients (n=285) followed in Centro Hospitalar São João with stage IIIB/IV NSCLC or with recurrence or progression at the time of the inclusion period, who were submitted to EGFR mutation screening, between October 2009 and June 2013. Criteria for screening were adenocarcinoma histology, specific characteristics as female sex, younger age or absence of smoking habits. Follow up for survival was censured in January 2016.
Study ethical approval was obtained by the Ethical Committee from the present Hospital.
DataAge at diagnosis, sex, smoking habits, tumor stage, histopathology, EGFR mutational status, treatments, overall survival and tumor progression were evaluated.
Tumor staging was based on the 6th TNM system in patients diagnosed until the end of 2010 and on the 7th TNM system from 2011 to June 2013. The stage considered was the tumor stage at diagnose. Regarding smoking habits, patients were classified as non-smokers, active smokers and ex-smokers (≥6 months of cessation). The overall survival was calculated using the difference between the date of death and the diagnosis date.
EGFR Mutation ScreeningThe EGFR mutation screening was performed by IPATIMUP (Institute of Molecular Pathology and Immunology of the University of Porto). The search of the exons 18–21 of the EGFR gene was performed through direct sequencing of Polymerase Chain Reaction (PCR) products obtained from the tumoral cells.
Statistical AnalysisDescriptive statistics (frequency, median and mean) were used to calculate the demographic and clinical characteristics. Categorical comparisons were calculated by chi-square test or by Fisher's exact test. Continuous variables were compared using the t-test.
The survival related results were obtained using the log-rank Kaplan–Meier product-limit estimates. Patients with one month or less of overall survival were excluded for the survival analysis. Statistical significance was set at P<.05 for all analyses. All analyses were performed using the software IBM SPSS (Statistical Package for the Social Sciences) v.21.
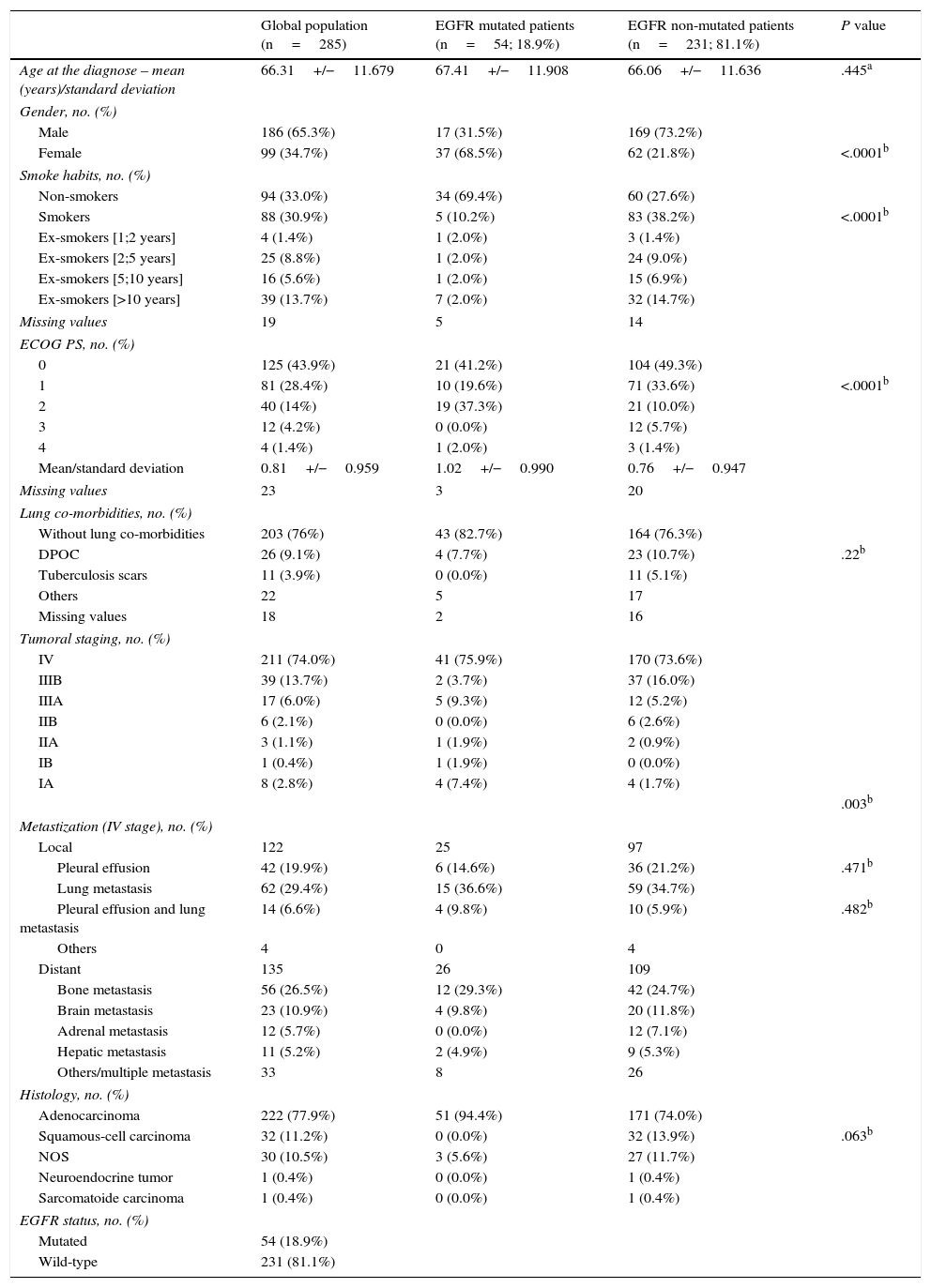
ResultsDemographic characteristics, clinical staging and histology are summarized in Table 1. Among the 285 patients included, 186 (65.3%) were male and 99 (34.7%) female. The mean age at diagnosis was 66.3 years (standard deviation 11.7 years). The majority of the patients had some degree of smoke exposure, 88 (30.9%) were smokers, 84 (29.3%) ex-smokers and 94 (33.0%) never smoked. Within ex-smokers, most (n=39; 46.4%) quit smoking over the last ten years before diagnosis.
Global, EGFR Mutated and EGFR Non-mutated Population Characterization.
| Global population (n=285) | EGFR mutated patients (n=54; 18.9%) | EGFR non-mutated patients (n=231; 81.1%) | P value | |
|---|---|---|---|---|
| Age at the diagnose – mean (years)/standard deviation | 66.31+/−11.679 | 67.41+/−11.908 | 66.06+/−11.636 | .445a |
| Gender, no. (%) | ||||
| Male | 186 (65.3%) | 17 (31.5%) | 169 (73.2%) | |
| Female | 99 (34.7%) | 37 (68.5%) | 62 (21.8%) | <.0001b |
| Smoke habits, no. (%) | ||||
| Non-smokers | 94 (33.0%) | 34 (69.4%) | 60 (27.6%) | |
| Smokers | 88 (30.9%) | 5 (10.2%) | 83 (38.2%) | <.0001b |
| Ex-smokers [1;2 years] | 4 (1.4%) | 1 (2.0%) | 3 (1.4%) | |
| Ex-smokers [2;5 years] | 25 (8.8%) | 1 (2.0%) | 24 (9.0%) | |
| Ex-smokers [5;10 years] | 16 (5.6%) | 1 (2.0%) | 15 (6.9%) | |
| Ex-smokers [>10 years] | 39 (13.7%) | 7 (2.0%) | 32 (14.7%) | |
| Missing values | 19 | 5 | 14 | |
| ECOG PS, no. (%) | ||||
| 0 | 125 (43.9%) | 21 (41.2%) | 104 (49.3%) | |
| 1 | 81 (28.4%) | 10 (19.6%) | 71 (33.6%) | <.0001b |
| 2 | 40 (14%) | 19 (37.3%) | 21 (10.0%) | |
| 3 | 12 (4.2%) | 0 (0.0%) | 12 (5.7%) | |
| 4 | 4 (1.4%) | 1 (2.0%) | 3 (1.4%) | |
| Mean/standard deviation | 0.81+/−0.959 | 1.02+/−0.990 | 0.76+/−0.947 | |
| Missing values | 23 | 3 | 20 | |
| Lung co-morbidities, no. (%) | ||||
| Without lung co-morbidities | 203 (76%) | 43 (82.7%) | 164 (76.3%) | |
| DPOC | 26 (9.1%) | 4 (7.7%) | 23 (10.7%) | .22b |
| Tuberculosis scars | 11 (3.9%) | 0 (0.0%) | 11 (5.1%) | |
| Others | 22 | 5 | 17 | |
| Missing values | 18 | 2 | 16 | |
| Tumoral staging, no. (%) | ||||
| IV | 211 (74.0%) | 41 (75.9%) | 170 (73.6%) | |
| IIIB | 39 (13.7%) | 2 (3.7%) | 37 (16.0%) | |
| IIIA | 17 (6.0%) | 5 (9.3%) | 12 (5.2%) | |
| IIB | 6 (2.1%) | 0 (0.0%) | 6 (2.6%) | |
| IIA | 3 (1.1%) | 1 (1.9%) | 2 (0.9%) | |
| IB | 1 (0.4%) | 1 (1.9%) | 0 (0.0%) | |
| IA | 8 (2.8%) | 4 (7.4%) | 4 (1.7%) | |
| .003b | ||||
| Metastization (IV stage), no. (%) | ||||
| Local | 122 | 25 | 97 | |
| Pleural effusion | 42 (19.9%) | 6 (14.6%) | 36 (21.2%) | .471b |
| Lung metastasis | 62 (29.4%) | 15 (36.6%) | 59 (34.7%) | |
| Pleural effusion and lung metastasis | 14 (6.6%) | 4 (9.8%) | 10 (5.9%) | .482b |
| Others | 4 | 0 | 4 | |
| Distant | 135 | 26 | 109 | |
| Bone metastasis | 56 (26.5%) | 12 (29.3%) | 42 (24.7%) | |
| Brain metastasis | 23 (10.9%) | 4 (9.8%) | 20 (11.8%) | |
| Adrenal metastasis | 12 (5.7%) | 0 (0.0%) | 12 (7.1%) | |
| Hepatic metastasis | 11 (5.2%) | 2 (4.9%) | 9 (5.3%) | |
| Others/multiple metastasis | 33 | 8 | 26 | |
| Histology, no. (%) | ||||
| Adenocarcinoma | 222 (77.9%) | 51 (94.4%) | 171 (74.0%) | |
| Squamous-cell carcinoma | 32 (11.2%) | 0 (0.0%) | 32 (13.9%) | .063b |
| NOS | 30 (10.5%) | 3 (5.6%) | 27 (11.7%) | |
| Neuroendocrine tumor | 1 (0.4%) | 0 (0.0%) | 1 (0.4%) | |
| Sarcomatoide carcinoma | 1 (0.4%) | 0 (0.0%) | 1 (0.4%) | |
| EGFR status, no. (%) | ||||
| Mutated | 54 (18.9%) | |||
| Wild-type | 231 (81.1%) | |||
Regarding clinical staging, most patients (n=250; 87.7%) were diagnosed at advanced stages of the disease and 35 (12.3%) presented with non-advanced stages with progression at the time of EGFR analysis.
Among stage IV patients, 72 (34.1%) had intrapulmonary metastasis, 85 (40.3%) distant metastasis and 50 (23.7%) both intrapulmonary and distant metastasis. The most frequent organs metastasized were bone (n=56; 26.5%), followed by brain (n=23; 10.9%), adrenal (n=12; 5.7%) and liver (n=11; 5.2%). Twenty-seven patients (12.8%) had more than one organ involved.
From the analysis of 285 lung tissue samples, 222 (77.9%) corresponded to adenocarcinoma, 32 (11.2%) to squamous cell carcinoma and 30 (10.5%) to not otherwise specified (NOS) NSCLC. There were sporadic samples (n=2, 0.8%) of neuroendocrine and sarcomatoid tumors.
The EGFR screening was made on samples obtained from transthoracic biopsies in 88 (30.9%) cases, bronchial biopsies in 84 (29.5%), surgical specimens in 29 (10.2%), pleural fluid in 26 (8.4%) and biopsies of metastasis in 18 (6.3%).
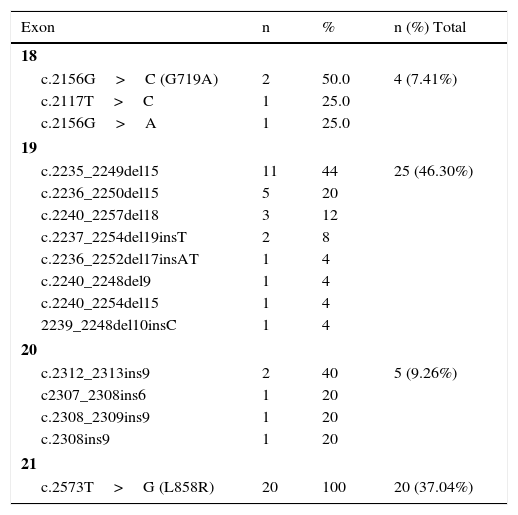
EGFR mutations were detected in 54 patients (18.9%), of which 25 (46.3%) were in exon 19, 20 (37.0%) in exon 21, 5 (9.3%) in exon 20, and 4 (7.4%) in exon 18 (Table 2). There were no samples with more than one mutation. The most common mutations were in-frame deletions at exon 19 (n=25; 46.3%) ant the p.L858R missense mutation in exon 21 (n=20; 37.1%).
EGFR Mutations.
| Exon | n | % | n (%) Total |
|---|---|---|---|
| 18 | |||
| c.2156G>C (G719A) | 2 | 50.0 | 4 (7.41%) |
| c.2117T>C | 1 | 25.0 | |
| c.2156G>A | 1 | 25.0 | |
| 19 | |||
| c.2235_2249del15 | 11 | 44 | 25 (46.30%) |
| c.2236_2250del15 | 5 | 20 | |
| c.2240_2257del18 | 3 | 12 | |
| c.2237_2254del19insT | 2 | 8 | |
| c.2236_2252del17insAT | 1 | 4 | |
| c.2240_2248del9 | 1 | 4 | |
| c.2240_2254del15 | 1 | 4 | |
| 2239_2248del10insC | 1 | 4 | |
| 20 | |||
| c.2312_2313ins9 | 2 | 40 | 5 (9.26%) |
| c2307_2308ins6 | 1 | 20 | |
| c.2308_2309ins9 | 1 | 20 | |
| c.2308ins9 | 1 | 20 | |
| 21 | |||
| c.2573T>G (L858R) | 20 | 100 | 20 (37.04%) |
In the EGFR mutated group there was a predominance of female versus male (P<.001) and of non-smokers versus smokers or ex-smokers (P<.001).
There was no statistically significant difference between groups regarding tumor progression. The most frequent de novo metastasis diagnosed in both groups were bone metastasis (n=33; 37.5%) followed by brain metastasis (n=27; 30.7%) and hepatic metastasis (n=20; 22.7%).
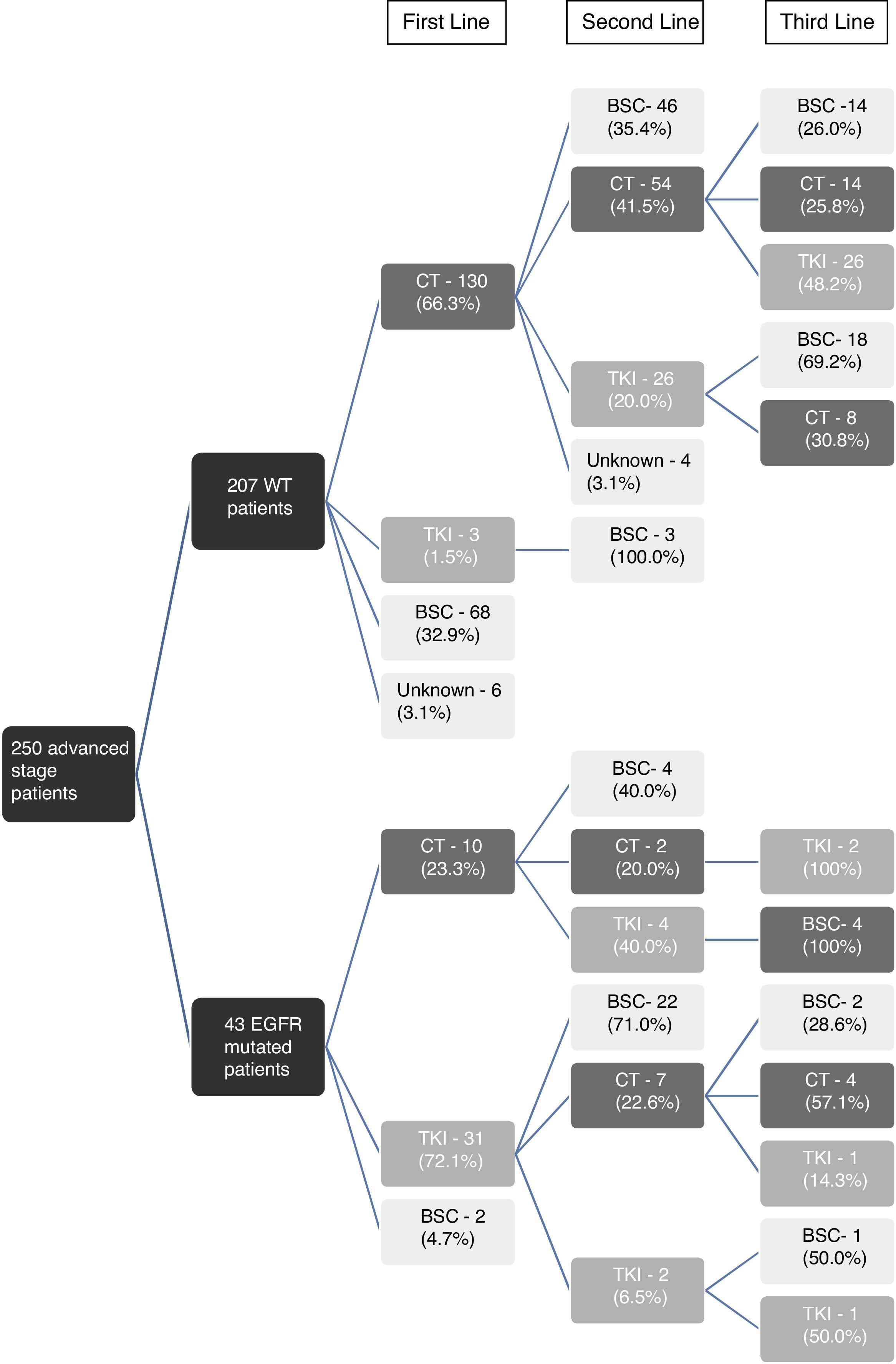
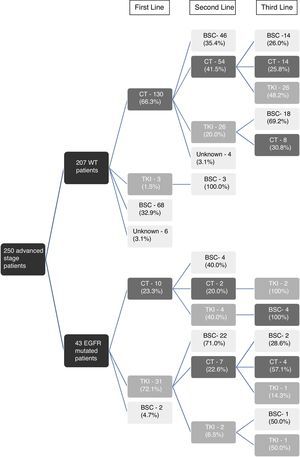
The first line treatment options, within the 43 EGFR mutated patients with advanced disease (79.6% of all EGFR mutant cases), were TKI in 31 (72.1%) patients, chemotherapy in 10 (23.3%) patients and best supportive care in 2 (4.7%) patients.
Regarding the TKI treatment, 37 (86.0%) EGFR mutant patients were exposed to TKI: 31 (83.8%) in first line of treatment, 4 (10.8%) in second line and 2 (5.4%) in third line (Fig. 1). The TKI more often used was Erlotinib in 21 (65.8%) patients and Gefitinib in 10 (27.0%). The mean duration of the TKI treatment was 8.8±7.7 months (median of 8 months).
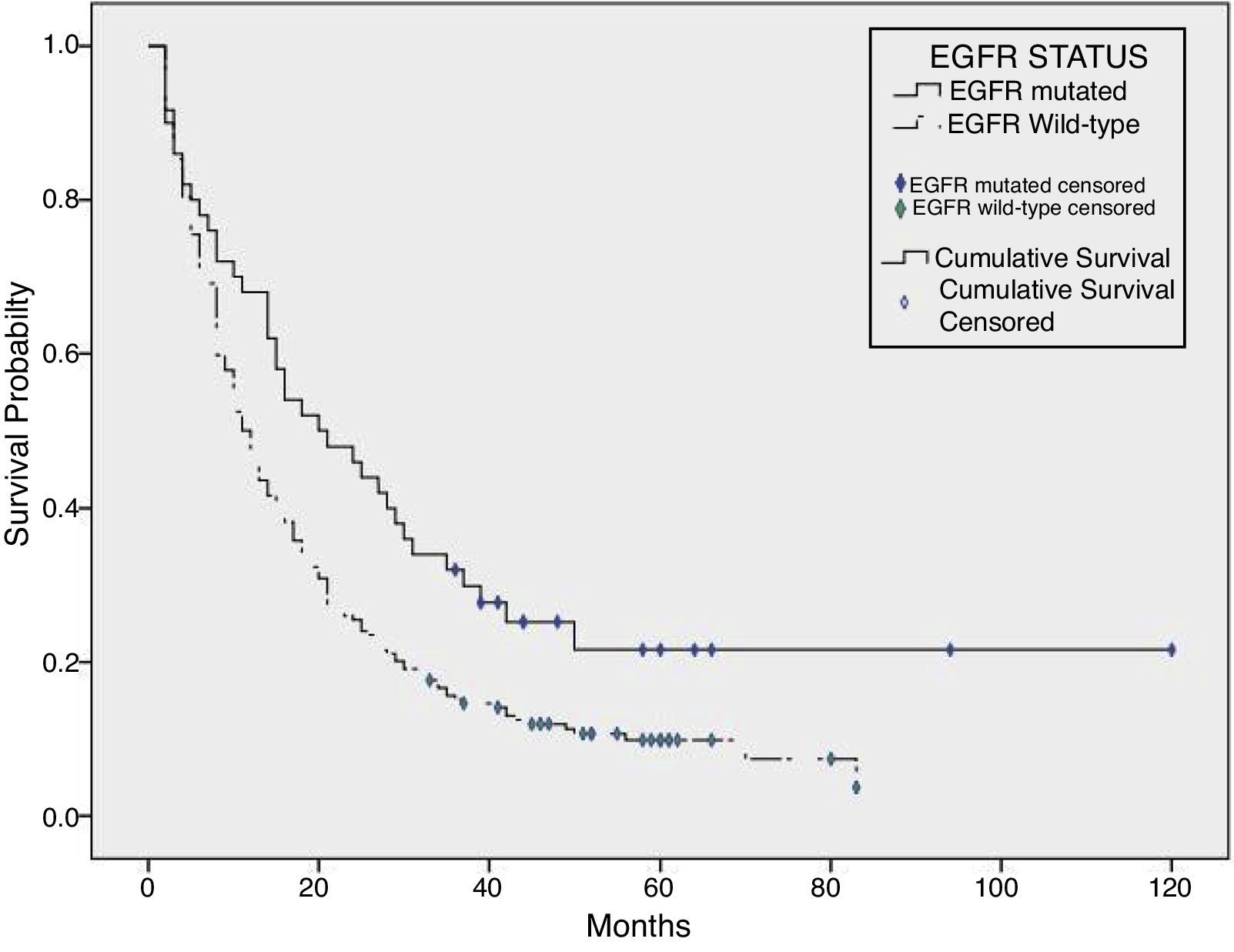
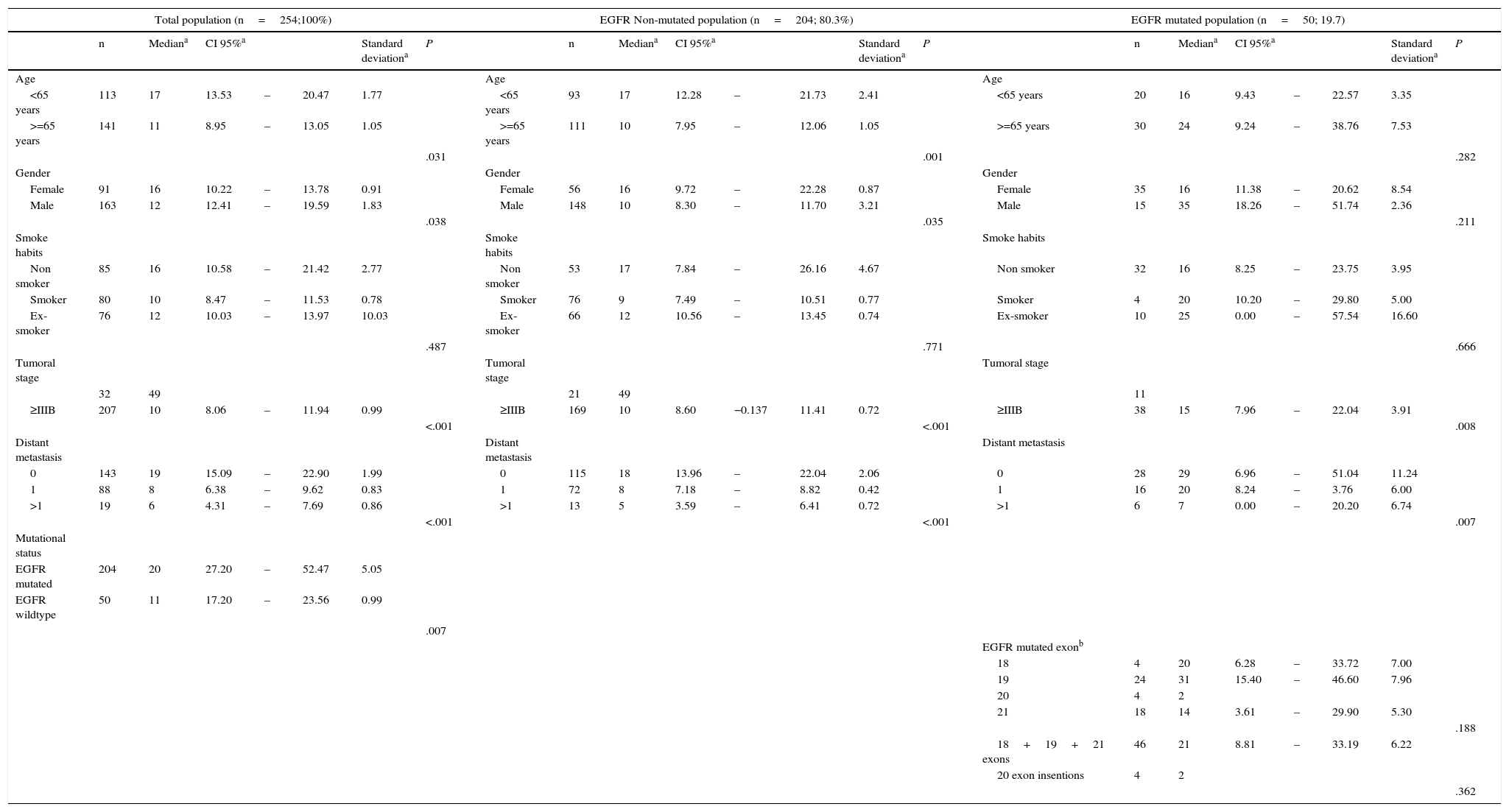
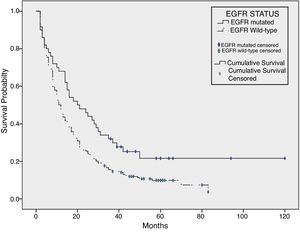
The global median overall survival (OS) was 12.0 months [95% confidence interval (CI) 9.6–14.4]. Analyzing the cohort by its EGFR mutational status, the median overall survival of the non-mutated group was 11.0 months (95% CI 9.1–12.9) and 20.0 months for the mutated group (95% CI 10.1–29.9) (P=.007) (Fig. 2).
In the non-mutated group, younger patients (<65 years; P<.001) and female sex (P=.03) had better overall survival (Table 3). Among the EGFR mutated group, sex (P=.342), age (P=.253), smoking habits (P=.666) and the EGFR exon mutated [KM comparison of 4 exons: P=.188, KM of TKI sensible mutations versus TKI non-sensible mutations (EGFR exon 20 insertion mutations): P=.362] did not have a statistical impact on OS.
Kaplan–Meier Global Survival Analysis.
| Total population (n=254;100%) | EGFR Non-mutated population (n=204; 80.3%) | EGFR mutated population (n=50; 19.7) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mediana | CI 95%a | Standard deviationa | P | n | Mediana | CI 95%a | Standard deviationa | P | n | Mediana | CI 95%a | Standard deviationa | P | |||||||||
| Age | Age | Age | |||||||||||||||||||||
| <65 years | 113 | 17 | 13.53 | – | 20.47 | 1.77 | <65 years | 93 | 17 | 12.28 | – | 21.73 | 2.41 | <65 years | 20 | 16 | 9.43 | – | 22.57 | 3.35 | |||
| >=65 years | 141 | 11 | 8.95 | – | 13.05 | 1.05 | >=65 years | 111 | 10 | 7.95 | – | 12.06 | 1.05 | >=65 years | 30 | 24 | 9.24 | – | 38.76 | 7.53 | |||
| .031 | .001 | .282 | |||||||||||||||||||||
| Gender | Gender | Gender | |||||||||||||||||||||
| Female | 91 | 16 | 10.22 | – | 13.78 | 0.91 | Female | 56 | 16 | 9.72 | – | 22.28 | 0.87 | Female | 35 | 16 | 11.38 | – | 20.62 | 8.54 | |||
| Male | 163 | 12 | 12.41 | – | 19.59 | 1.83 | Male | 148 | 10 | 8.30 | – | 11.70 | 3.21 | Male | 15 | 35 | 18.26 | – | 51.74 | 2.36 | |||
| .038 | .035 | .211 | |||||||||||||||||||||
| Smoke habits | Smoke habits | Smoke habits | |||||||||||||||||||||
| Non smoker | 85 | 16 | 10.58 | – | 21.42 | 2.77 | Non smoker | 53 | 17 | 7.84 | – | 26.16 | 4.67 | Non smoker | 32 | 16 | 8.25 | – | 23.75 | 3.95 | |||
| Smoker | 80 | 10 | 8.47 | – | 11.53 | 0.78 | Smoker | 76 | 9 | 7.49 | – | 10.51 | 0.77 | Smoker | 4 | 20 | 10.20 | – | 29.80 | 5.00 | |||
| Ex-smoker | 76 | 12 | 10.03 | – | 13.97 | 10.03 | Ex-smoker | 66 | 12 | 10.56 | – | 13.45 | 0.74 | Ex-smoker | 10 | 25 | 0.00 | – | 57.54 | 16.60 | |||
| .487 | .771 | .666 | |||||||||||||||||||||
| Tumoral stage | Tumoral stage | Tumoral stage | |||||||||||||||||||||
| 32 | 49 | 21 | 49 | 11 | |||||||||||||||||||
| ≥IIIB | 207 | 10 | 8.06 | – | 11.94 | 0.99 | ≥IIIB | 169 | 10 | 8.60 | −0.137 | 11.41 | 0.72 | ≥IIIB | 38 | 15 | 7.96 | – | 22.04 | 3.91 | |||
| <.001 | <.001 | .008 | |||||||||||||||||||||
| Distant metastasis | Distant metastasis | Distant metastasis | |||||||||||||||||||||
| 0 | 143 | 19 | 15.09 | – | 22.90 | 1.99 | 0 | 115 | 18 | 13.96 | – | 22.04 | 2.06 | 0 | 28 | 29 | 6.96 | – | 51.04 | 11.24 | |||
| 1 | 88 | 8 | 6.38 | – | 9.62 | 0.83 | 1 | 72 | 8 | 7.18 | – | 8.82 | 0.42 | 1 | 16 | 20 | 8.24 | – | 3.76 | 6.00 | |||
| >1 | 19 | 6 | 4.31 | – | 7.69 | 0.86 | >1 | 13 | 5 | 3.59 | – | 6.41 | 0.72 | >1 | 6 | 7 | 0.00 | – | 20.20 | 6.74 | |||
| <.001 | <.001 | .007 | |||||||||||||||||||||
| Mutational status | |||||||||||||||||||||||
| EGFR mutated | 204 | 20 | 27.20 | – | 52.47 | 5.05 | |||||||||||||||||
| EGFR wildtype | 50 | 11 | 17.20 | – | 23.56 | 0.99 | |||||||||||||||||
| .007 | |||||||||||||||||||||||
| EGFR mutated exonb | |||||||||||||||||||||||
| 18 | 4 | 20 | 6.28 | – | 33.72 | 7.00 | |||||||||||||||||
| 19 | 24 | 31 | 15.40 | – | 46.60 | 7.96 | |||||||||||||||||
| 20 | 4 | 2 | |||||||||||||||||||||
| 21 | 18 | 14 | 3.61 | – | 29.90 | 5.30 | |||||||||||||||||
| .188 | |||||||||||||||||||||||
| 18+19+21 exons | 46 | 21 | 8.81 | – | 33.19 | 6.22 | |||||||||||||||||
| 20 exon insentions | 4 | 2 | |||||||||||||||||||||
| .362 | |||||||||||||||||||||||
Tumor stagingP=.005) and non-mutated (P<.001) populations.
The two-year overall survival of global population was 26.7%, corresponding to 75 patients (26.7%), 52 (20.6%) non-mutated patients and 23(42.6%) EGFR mutated. At the end of follow-up (January 2016), 32 (11.2%) patients were alive [12 (22.2%) EGFR mutated and 20 (8.7%) non-mutated patients].
DiscussionTo our best knowledge, this is one of the first overall survival analysis, outside of clinical trials, comparing EGFR mutated patients to EGFR non-mutated patients with lung cancer. Our results showed a frequency of EGFR mutation of 18.9% and better overall survival in this population.
The mutation distribution was predominantly in the exons 19 and 21 [25 (46.3%) and 20 (37.0%) mutations, respectively], totalizing 45 (83.3%) of all mutations. 5 (9.3%) mutations occurred in exons 20 and 4 (7.4%) in exon 18. This distribution is similar to other studies.17,18
The frequency of EGFR mutations varies geographically. Higher frequencies are found in Asia, as shown in the work of Shi et al. (51.4%) that represents several regions of that continent.19 In the Latin America intermediate frequencies are 33.2% in Argentina, Colombia, Mexico and Peru.20 In the European region, studies revealed a frequency of 16.6%, 12.3% and 4.9% in Spain,10 Denmark21 and Germany.22
The frequency of EGFR mutations in the Portuguese population is undetermined. In the study of Mello et al., the frequency of this mutation was 16.9%17 while in Castro et al. the global frequency was 13.1%.23 Still, in this last work, different frequencies were determined: 16.3% and 10.4% corresponding to different inclusion criteria, from 2006 to 2009 were included patients with adenocarcinoma or without smoking habits, while in 2010 all patients with NSCLC were included.23 Our mutation frequency of 18.9% is slightly higher to those published. Some bias related to phenotypic preselection could have occurred. The majority were stage III/IV adenocarcinoma but some patients were selected based on their characteristics such as non-smokers, female sex, younger age and progression or recurrence of tumor staged
Association between EGFR mutation status and survival is difficult to estimate, particularly outside of a clinical trial setting. The obstacle to this association could be explained by the different lines of treatment and the crossover of treatments.24,25 The median OS of the EGFR mutated group was 9 months superior to the OS of the non-mutated group (20.0 vs 11.0 months; P=.007). This values for OS are similar to other clinical trials,16,24,26–28 particularly in the EURTAC trial. These OS difference could be explained by a possible influence of the mutational status in the prognosis and by the use of more efficacious drugs than the usual chemotherapy in the mutated population.
Some studies, as in the Iressa Pan-Asia Survival Study (IPASS), comparing gefitinib with paclitaxel plus carboplatin as the first-line therapy in Asian patients, have demonstrated a statistically significant higher response rate to chemotherapy in EGFR mutated patients (47.3% versus 23.5%) than patient without EGFR mutations, but the progression-free survival (PFS) and OS were not different between patients with and without EGFR mutation in the chemotherapy arm.27 In previous studies, the survival of EGFR mutated patients was significantly longer than those without EGFR mutations in groups of patients who received chemotherapy alone without gefitinib or erlotinib.29,30 The study of Lin CC et al.31 corroborated that EGFR mutations are associated with a higher tumor response rate to chemotherapy, but are not a predictive biomarker for PFS and OS. Applying the Kaplan–Meier product-limit estimates (Table 3) in the global population, the factors that were associated with a better OS were age inferior to 65 years, female sex, tumor stage
In the non-mutated population features like age <65 years, female sex and stage
Results regarding clinical factors that may influence prognosis also vary across studies. Some authors report an association between extrathoracic metastasis, in particular brain metastasis, and the presence of L858 mutation with worse survival.32–34
In this study the relevance of the molecular study was confirmed, permitting the identification of the EGFR mutated patients who have a distinct clinical behavior regarding overall survival and response to TKI.
The decision to screen for EGFR mutation was influenced by adenocarcinoma histology, female gender and non-smoking status. This selection bias represents a major limitation of the present study. Within the EGFR mutated group there were only a small group of patients with non-sensible TKI mutations (n=5). For overall survival calculations, the small size of this group did not permit any conclusion in particular. Those patients were considered within the rest of the EGFR mutated patients which could represent a limitation for the study results. The study reflects the clinical results and therefore has inherent limitations such its retrospective character and a small population analyzed, but its strength is that it is one of the first real life studies aiming the OS of an EGFR mutated population.
Our data showed a better overall survival for EGFR activating mutated patients independently of the clinical characteristics, suggesting that it can be a favorable prognostic marker.
AuthorshipFilipa Aguiar: study conception and design, data collection, data analysis and interpretation, statistical analysis, manuscript writing.
Gabriela Fernandes: study conception and design, data collection, data analysis and interpretation, manuscript writing; manuscript review.
José Carlos Machado and Luis Cirnes: EGFR mutation screening, manuscript review.
Conceição Souto Moura: pathology analysis, manuscript review.
Venceslau Hespanhol and Henrique Queiroga: study supervision, manuscript review.
Conflict of InterestThe authors declare no conflict of interest.