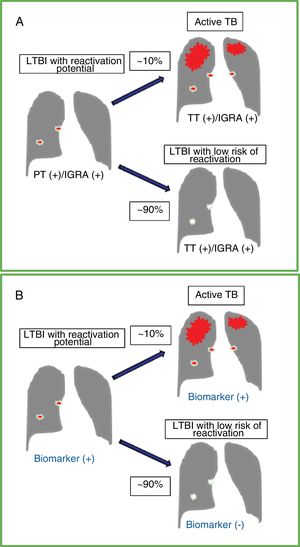
Accurate detection and risk stratification of latent tuberculous infection (LTBI) remains a global clinical and public health challenge. It is estimated that one quarter of the world's population is infected with Mycobacterium tuberculosis (MTB), mostly in asymptomatic and latent forms.1 Immunocompetent individuals with LTBI have a 5 %–10% risk of developing active tuberculous disease (TB) during their lifetime (Fig. 1A). This risk increases in the immunosuppressed population, although it decreases substantially with preventive therapy.2 In recent years there have been important scientific advances in both pathophysiological understanding and the diagnosis and management of LTBI (see the editorial New Perspectives in Latent Tuberculous Infection).3 However, laboratory tests currently available for the detection of LTBI have serious diagnostic limitations, including a predictive value of < 5% for identifying subjects with LTBI who will develop disease reactivation.4 Tuberculin testing and interferon-gamma release assays can detect cell-mediated immune responses to MTB. However, none of these tests can differentiate between individuals who do not develop infection after exposure to the bacillus (innate immune response) or those who achieve subsequent eradication and/or effective bacillary containment (adaptive immune response with calcified granuloma) from others who have silent and/or persistent infection and a high risk of reactivation.5 Therefore, diagnostic tests need to be refined, not only to optimize their detection capacity, but also to better assess the risk of reactivation (Fig. 1B), thereby improving the selection of individuals who can really benefit from preventive treatment and helping improve TB eradication levels in many parts of the world, including Spain and Latin America.6–8
Natural course of latent tuberculous infection (LTBI), diagnostic tests and the ideal biomarker. A. Course of an untreated LTBI in an immunocompetent individual with a 5%-10% risk of developing active tuberculosis (TB) over time. The tests available for LTBI, both tuberculin testing (TT) and interferon-gamma release (IGRA) assays, usually give positive results before and after reactivation of active TB. B. Results of a test with an ideal biomarker that can differentiate latent infection with reactivation potential versus an individual with positive TT and/or IGRA results but with no significant risk of reactivation. An ideal marker would increase significantly as the disease progresses from LTBI with reactivation potential to symptomatic TB.
Recently, longitudinal studies in individuals with risk factors for TB and controlled studies in primate LTBI models have helped clarify temporospacial aspects of pathophysiology in LTBI and progression to TB.9–11 Immune response to MTB is known to be complex, dynamic, and multifocal. This is a process in which different immune system cells participate by forming granulomas in infected organs and by activating regional nodes in order to contain the infection and potentially eradicate it. However, these mechanisms might fail, allowing the infection to progress.9–11 These studies also show that not only do effector CD4 T cells and activated macrophages control bacterial replication and prevent disease progression or reactivation, but that other immune cells also play a leading role.9,11 However, the molecular mechanisms of immunological evasion and tolerance are not fully understood.10 A limitation of prospective studies of this type that involve large numbers of individuals, immunological tests and RNA signatures, is that they are difficult to replicate because of the high cost and sophisticated technologies required to both perform them and analyze the data.11
Another critical area in LTBI is the development of markers of response to preventive therapy.4 An accurate diagnostic test with a reliable biomarker for detecting this type of therapeutic response would be of great clinical and public health value, especially in areas of high incidence where recurrent exposure and potential reinfection can be a common phenomenon. Moreover, immunological biomarkers of response to preventive LTBI treatment would also be indicators of an immune status with a low risk of progression to TB, since preventive treatment is associated with a greater than 60% reduction in the risk of reactivation.12,13 The difficulty in identifying high- and low-risk biomarkers in LTBI lies in the lack of a gold standard that accurately indicates the existence of a latent infection with reactivation potential. What we know clinically as LTBI is a term that encompasses a heterogeneous group of individuals at different stages of infection, including reactivatable infections (LTBI with reactivation potential) and individuals with an immune response to MTB antigens (tuberculin test and/or interferon-gamma release assay) with eradicated bacilli or with no possibility of reactivation, as in the case of most calcified granulomas (LTBI with low risk of reactivation) (Fig. 1). In this setting, a research strategy that assesses the effect of preventive treatment on the different LTBI subgroups may reveal markers of LTBI with reactivation potential (Fig. 2). Using this strategy in a combinatorial approach, T-lymphocyte-specific antigen response markers [CD25 (IL-2 receptor α-chain) and CD134 (OX40, a TNF-α receptor) co-expression] could statistically differentiate individuals with untreated LTBI from those with a history of receiving preventive treatment.14 In a secondary analysis using a multidimensional model to estimate LTBI risk, individuals with positive results in both interferon-gamma release assays and co-expression of these MTB-specific antigenic biomarkers in CD4+ and CD8+ T cells had the greatest risk of developing TB reactivation.14 A similar strategy also showed a reduction in the proportion of T cells expressing the activation marker CD38 in individuals treated for LTBI.15 This research strategy has limitations, namely: the heterogeneous study group; the dynamics and time in which these markers are modified with treatment; and the dependence on an adequate immune function and without anergy. Despite this, the strategy will be useful not only for the identification and preliminary validation of biomarkers of treatment response, which could subsequently be studied using more conclusive longitudinal validation, but also for LTBI risk assessment.7,15 Markers that rapidly decrease with LTBI treatment and are MTB-specific and reproducible in different populations will most reliably identify individuals who respond to treatment.
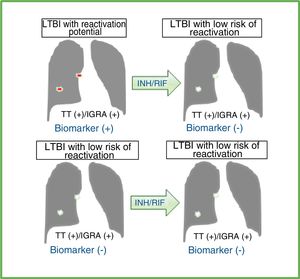
Effect of preventive treatment on diagnostic tests and ideal biomarkers in individuals with latent tuberculous infection (LTBI) with reactivation potential or low risk of reactivation.
Effect of preventive treatment with isoniazid (INH) and/or rifampicin (RIF) on tuberculin test (TT) results and/or interferon-gamma release assays (IGRA) in individuals with a diagnosis of LTBI. The available diagnostic tests do not distinguish individuals with LTBI with reactivation potential from those who have already bacteriologically eradicated or effectively and permanently contained tuberculous infection (LTBI with low risk of reactivation). An ideal biomarker should be detected prior to preventive LTBI treatment and not subsequently in cases of LTBI with reactivation potential. In cases of LTBI with low risk of reactivation, the ideal marker should not be detected before or after treatment. This does not occur with TT and/or IGRA results, which are usually positive after preventive treatment. Therefore, an ideal biomarker would improve the selection of individuals who would actually benefit from LTBI treatment, thus avoiding preventive treatment in individuals who do not need it or who have a significant risk of developing side effects with these antibiotics.
In conclusion, new biomarkers may be discovered and evaluated in the future to stratify the risk of individuals with LTBI and their response to treatment, but prospective longitudinal studies with different types of populations are still needed to validate these promising tests around the world.
Conflict of interestsThe content of this document is the sole responsibility of the authors and does not necessarily represent the official views of the National Institute of Health of the United States of America, the Mayo Clinic, or any other organization. The authors received no financial or material support for this article. Dr. Escalante and his institution have filed patents related to immunodiagnostic laboratory methodologies for latent tuberculous infection. To date, no income or royalties have been received in association with these patents. None of the authors has any other conflict of interest to declare.
Dr. Escalante's research and academic work has been supported by grants and internal funds from the Mayo Clinic, including the “2019 Division of Pulmonary and Critical Care Medicine Midcareer Development Award”, and recently by funding from the National Institute of Health of the United States of America (NIH, #1R01AI141591).
Please cite this article as: Escalante P, Arias-Guillén M, Palacios Gutiérrez JJ. Nuevos enfoques en investigación de la infección tuberculosa latente. Arch Bronconeumol. 2021;57:151–153.