The history of non-invasive mechanical ventilation goes back more than 100 years, but it was not until 1987 when what we could call “modern” non-invasive mechanical ventilation was developed. The description of Delaubier and Rideau of a patient with Duchenne's disease who had been effectively ventilated through a nasal mask marked the start of a new era in the history of non-invasive mechanical ventilation. Over these last 25years, we have witnessed exponential growth in its use, field of activity and technological advances on an exciting fast-paced track. We believe that it is time to review the main milestones that have marked the development of non-invasive mechanical ventilation to date, while paying homage to this therapeutic method that has contributed so much to the advancement of respiratory medicine in the last 25years.
La historia de la ventilación mecánica no invasiva se remonta más de 100años en el tiempo, pero no fue hasta 1987 cuando entramos en lo que podemos llamar la ventilación mecánica no invasiva moderna. La descripción de Delaubier y Rideau de un paciente con enfermedad de Duchenne ventilado eficazmente a través de una mascarilla nasal marcó el inicio de una nueva era en la historia de la ventilación mecánica no invasiva. En estos 25años hemos asistido a un crecimiento exponencial de su utilización, de su campo de actuación y de su desarrollo tecnológico, en una carrera apasionante y vertiginosa. Consideramos que es oportuno realizar una revisión de los principales hitos que han marcado el desarrollo de la ventilación mecánica no invasiva hasta llegar al momento actual, y aprovechamos para rendir tributo a esta modalidad terapéutica que tanto ha contribuido al desarrollo de la neumología en los últimos 25años.
It has been 25 years since the use of positive pressure ventilation with non-invasive interfaces became standardized. These have been 25 exciting years in which a succession of important events has significantly changed the history of pulmonology and contributed to the advancement and expansion of respiratory medicine. It is time to review the progress of non-invasive mechanical ventilation (NIMV) over this period.
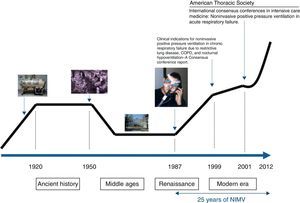
If the history of NIMV were plotted linearly, something similar to that shown in Fig. 1 would be obtained. Just like a traditional history book, Ancient History, the Middle Ages, the Renaissance and the current or Modern Era can all be identified along this line. Nevertheless, NIMV is not a new technique. Its history and origins go back more than 100 years and are linked to the invention of the iron lung by Drinker and Shaw in 1927. This marks the beginning of the Ancient History of NIMV. The iron lung was used for the first time in 1928 in the Boston Children's Hospital (MA, USA) in an unconscious girl with respiratory problems. Her rapid recovery helped popularize the so-called “Drinker Respirator”. Some years later, Emerson improved the Drinker prototype, marketing an iron lung that was smaller, cheaper, lighter, more silent and more reliable than its predecessor. He was accused of infringing Drinker's patents, but the complaints were not upheld and hospitals were filled with Emerson's iron lung.1 It played a crucial role during the poliomyelitis epidemics of the 1940s and 50s, keeping alive the 10% of polio patients who presented with acute respiratory failure and sustained ventilatory dependence.2 To avoid patients being shut away indefinitely in an institution, various hospitals decided to try to set up domiciliary mechanical ventilation programs. Iron lungs and their variants (ponchos and cuirasses) were the basic elements that supported the early home mechanical ventilation (HMV) programs.3
In the 1950s, a series of events took place that limited the use of the iron lung. The eradication of poliomyelitis thanks to generalized vaccination programs, along with the surge in positive pressure mechanical ventilation administered with respiratory tract intubation, effectively led to the disappearance of the iron lung.4 At this point, we enter the NIMV Middle Ages. However, just as in the Middle Ages, when wisdom and knowledge were restricted to the monasteries, there were certain places where the NIMV flame continued to burn and many patients continued to benefit from negative pressure ventilation. One of these “ventilation monasteries” is the Hospital María Ferrer in Buenos Aires, which is still active today and survivors from those times can still be seen living in iron lungs. The room of one of these patients can be seen in Fig. 2.
From the 1960s until 1988, 990 cases of patients receiving home ventilation in various countries were reported, principally in France,5 the United Kingdom6 and the United States.7 Fourteen percent (14%) of these patients used negative pressure equipment (iron lung or poncho), 70% used volumetric respirators with tracheotomy and in the remaining 16% the airway was accessed via mouth or nose pieces.8 A classic study in the field of home ventilation, published by Robert et al.9 in 1983, reported 222 patients home-ventilated with tracheotomy since 1960, providing a survival curve for patients pooled according to disease type that was used as a reference throughout the years.
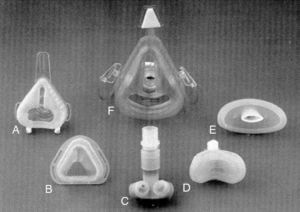
In this modest setting of patients ventilated at home, primarily via tracheotomy, and to a lesser extent, in a non-invasive manner with negative pressure equipment or mouthpieces, Delaubier and Rideau introduced the technique of intermittent positive pressure ventilation via a nasal mask in patients with muscular dystrophy that they called NIPPV (non-invasive positive pressure ventilation). These French authors showed that a patient with Duchenne muscular dystrophy could be adequately ventilated with a nasal mask designed by Sullivan in 1980 for applying continuous pressure support to patients with sleep apnea.10 The development of the first masks with which NIMV began to be applied was, as such, very closely associated with the development of continuous positive airway pressure (CPAP) for sleep apnea syndromes (Fig. 3). An article published in 1987 in the journal Agressologie11 was a historical milestone and a turning point in the history of NIMV, marking the transition between the Middle Ages and the Renaissance.
Non-invasive mechanical ventilation masks. (A) standard nasal mask (Respironics Inc., Murrysville, PA); (B) Sullivan nasal mask (Sullivan Bubble Mask, ResCare, San Diego, CA); (C) nasal pillows (Nellcor Puritan Bennett, ADAM circuit, Pleasanton, CA); (D) mini mask (Respironics Inc., Murrysville, PA); (E) lip seal (Nellcor Puritan Bennett, Pleasanton, CA); (F) total-face mask (Spectrum, Respironics Inc., Murrysville, PA).
The confirmation that mechanical ventilation could be efficacious, comfortable and well-tolerated with the use of a nasal mask added to the exponential growth of patients on long-term ventilation in their own homes and the development of positive pressure NIMV in most respiratory medicine wards. Negative pressure ventilation techniques were practically abandoned and their use has been exceptional ever since.
NIMV spread rapidly as the treatment of choice in patients with respiratory failure due to neuromuscular diseases, rib-cage defects and the sequelae of tuberculosis, which became known as restrictive respiratory failure, due to the spirometric and radiological characteristics of these patients. Other causes of hypoventilation, such as obesity, were added to the list of indications for NIMV.12,13 These patients were adapted to volumetric ventilators in a hospital setting and then sent home, and a new offer of domiciliary respiratory therapy appeared alongside oxygen therapy in the portfolio of treatment service providers: HMV delivered by mask. The number of patients treated with mechanical ventilation at home vastly exceeded the estimations made by Estopá in 1996,14 who calculated that by the year 2000 in Europe, over 7000 patients would be receiving home mechanical ventilation. This brings us to the heart of modern NIMV history.
It is essential to mention the study by Leger et al., published in 1994,15 describing the first series of NIMV patients treated at home, with a 5-year follow-up of 276 patients. The results show the benefit in terms of survival provided by NIMV in patients with neuromuscular diseases, kyphoscoliosis and sequelae of tuberculosis, and to a lesser extent, patients with chronic obstructive pulmonary disease (COPD) and bronchiectasis.
Restrictive respiratory failure, volumetric ventilators, the nasal mask, adaptation of patients in hospital and the use of nocturnal pulse oximetry as a basic monitoring tool were the cardinal points of NIMV in those early stages of modern NIMV, which culminated in 1999 in the publication in the journal Chest16 of the Consensus Conference on the clinical indications for non-invasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD and nocturnal hypoventilation. This article established the criteria for indicating home NIMV which even today remain applicable to a large extent. The 1990s were the decade of NIMV and HMV.17
In the year 2000, De Lucas et al.18 analyzed HMV in Spain and demonstrated the variability in its use across this country, with a prevalence of patients ventilated at home ranging between 0.93/100000 in the region of Castilla-La Mancha and 10.11/100000 in the Community of Madrid. At that time, 1821 patients were receiving HMV in Spain, which was initiated here in 1987 by the Hospital de Bellvitge (Barcelona). This article documents the implementation of the HMV program in the Hospital San Pedro de Alcántara (Cáceres) in 1988, in the Hospital Clínico Universitario in Valencia in 1990 and in the hospitals La Paz (Madrid), Gregorio Marañón (Madrid) and Son Dureta (Mallorca) in 1992. In the year 2000, the number of hospitals offering HMV programs reached 43.18 Two excellent reviews carried out by what were considered the pioneer groups in Spain, one on NIMV8 and one on HMV,19 were published in 1994, gathering all the knowledge in this area at the time in two articles which would become references for the groups contributing to the advancement of NIMV in Spain during the 1990s. Some years later, a European study, EUROVENT, analyzed data from 16 countries and more than 20000 patients, pointing out, like the Spanish study, a wide variability in indications and types of ventilators and interfaces used in the various countries studied. The prevalence of patients included in HMV programs also differed, with figures of 0.1/100000 in Poland compared to 17/100000 in France, with a mean prevalence of 6.6/100000.20
In 1992, BiPAP®, a ventilator originally designed for the treatment of patients with sleep apnea-hypopnea syndrome who could not tolerate the high pressures of CPAP, was introduced into the market by Respironics. With the philosophy of reducing pressure during expiration and intermittently reducing pressurization in the circuit, it rapidly became a new ventilatory modality in the field of NIMV: bi-level pressure ventilation.21 In addition to the concepts of tidal volume, I/E ratio, respiratory rate, high and low pressure alarms and trigger, all normal parameters in volumetric ventilators, other terms, such as pressure support, inspiratory positive airway pressure (IPAP), expiratory positive airway pressure (EPAP) and positive end-expiratory pressure (PEEP), had to be assimilated, along with the concept that respiratory rate and I/E ratio was controlled by the patient himself, the trigger depended on the ventilator and the tidal volume was the result of all these factors and could not be determined previously by the physicians. Moreover, these ventilators did not have batteries or alarms (Fig. 4). Leaving aside the enthusiasm of some respiratory medicine specialists, it is really easy to understand how the appearance in the intensive care unit (ICU) of one of these tiny devices for transferring a complex patient labeled as difficult-to-wean to the general hospital ward would generate certain distrust in the eyes of intensive care physicians. In 1996, the following was published: “Recently we have begun to use the device known as BiPAP. This system should be called pressure support, and generates pressure through a constant airflow. […] There is still no general consensus on the usefulness of these devices, but in view of their simplicity and the low cost compared to volumetric ventilators, there is no doubt that they have a role in the area of home mechanical ventilation”,14 as was subsequently proven.
In 1995, Brochard et al.22 published a study in the New England Journal of Medicine showing that applying pressure support to patients with exacerbations of COPD with respiratory acidosis reduced the need for orotracheal intubation, ICU admission, hospital mortality and mean length of stay, and improved blood gases and pH. A few years later, bi-level pressure NIMV had become the gold standard for the treatment of this type of patient.23 The Global Initiative for Chronic Obstructive Lung Diseases (GOLD) established the criteria for this indication with level A scientific evidence.24 In 2002, the British Thoracic Society Standards of Care Committee established an incidence of 70 patients with COPD treated with NIMV per 250000 population per year.25 Plant et al.,26 for their part, clarified which patients could be treated in the general hospital ward and which could not. Other authors concerned themselves with determining the predictive factors for success in the acute patient setting, thus placing blood gases firmly in the awareness of physicians everywhere when initiating ventilation.27,28 Evidence of the efficacy of NIMV emerged, not only in patients with COPD, but also in other situations of acute respiratory failure, both hypercapnic and hypoxemic,29 and the emphasis of NIMV use switched from the chronic patient to the acute patient, from the volumetric ventilator to the bi-level pressure ventilator, from the nose mask to other interfaces with greater facial cover (oronasal, total, helmet) and from the home to the hospital. In 2001, the American Thoracic Society published the International Consensus Conference on NIMV in acute respiratory failure,30 which was a new historical milestone establishing recommendations which have served as guidelines for other standards and subsequent revisions.31,32
New fronts have been opened in the NIMV revolution in recent years. One of these has been the location in which patients should be treated. Far from the initial binomial of the respiratory medicine ward or ICU, the concept of intermediate respiratory care units has emerged, although this has not spread as widely as might be desired.33,34 It has become clear that NIMV in acute patients requires a specific infrastructure and specially dedicated personnel with the appropriate training, experience and 24-h availability.35 The increased complexity of ventilated patients and the greater investment in nursing time required for their care has produced significant changes in the organization of pulmonology departments.36 Patient care has gone beyond these units, and experience is growing in the successful application of NIMV in hospital and out-of-hospital emergency departments.37 The type of home-ventilated patients has expanded to include acute respiratory failure survivors, those impossible to wean and patients with frequent exacerbations. In parallel, NIMV is increasingly demanded for the growing number of hospitalized patients with hypercapnic respiratory failure in situations with a lower level of evidence, related with advanced progressive stages of many diseases and degenerative processes. NIMV and HMV are increasingly proposed in elderly patients, patients with a do-not-intubate order or with serious concomitant diseases, and with palliative intent; data are available on its utility in these critical situations.29
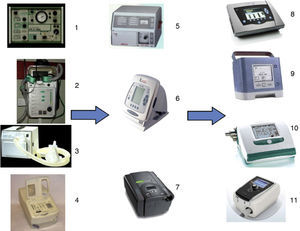
From a technological point of view, ventilators have evolved considerably with the progressive incorporation, depending on the manufacturer, of different ventilatory modes, sophisticated alarms, functions for regulating trigger, cycling, volumes and pressures, ability to ensure or guarantee volumes, and, more recently, the provision of monitoring tools. Screens with FiO2 curves, estimations, measurements or regulation, and integrated recording of hemoglobin saturation or expired CO2 are only some of the possibilities offered by latest-generation ventilators, thus making NIMV a little more complex (Fig. 5).
Non-invasive mechanical ventilation (NIMV) ventilators. Early NIMV ventilators: (1) Monnal D (Vitalaire); (2) Airox Home1 (France); (3) Bipap S/T (Respironics Inc., Murrysville, PA); (4) Harmony (Respironics Inc., Murrysville, PA). Second generation NIMV ventilators: (5) PV 501 (Breas Medical); (6) VIVO 40 (Breas Medical); (7) Synchrony (Respironics Inc., Murrysville, PA). Modern NIMV ventilators: (8) VIVO 50 (Breas Medical); (9) Trilogy 100 (Respironics Inc., Murrysville, PA); (10) Elisee 150 (ResMed SA); (11) Stellar 150 (ResMed SA).
NIMV also has its limitations. Against the setting of the uncontrolled expansion of its use, discussions are often raised about whether or not it should be initiated in critical situations, thus highlighting ethical dilemmas in an area with little scientific evidence and doubtful verifiable experience of the medical team.38 Similarly, the scarcity of standardized training and education in ventilation and the wide variability of the existing organizational models combined with a lack of data on the resources necessary for applying NIMV are other aspects highlighting the limitations of NIMV. Questions about whether or not a duty pulmonologist is required, if ventilation procedures should be concentrated in a specific hospital area or if the patients should be distributed according to their underlying disease, all await response.
It has been 25 years since that patient with Duchenne muscular dystrophy was successfully ventilated with a nasal mask and made known in the literature, and 25 years since the first patient was ventilated at home in Spain. The history of modern NIMV has been exciting and fast-paced. All the initial predictions have been surpassed. Nowadays young doctors have to deal with NIMV with top-of-the-range ventilators, often without the necessary historical perspective that everyone working with NIMV should have. What does the future hold for NIMV? Technological developments, monitoring, patient types, indications, infrastructures, new ventilatory modes and telemedicine all have to be taken on board. In coming years, research will focus on areas such as patient-ventilator asynchrony and sleep disorders in NIMV patients. For now, we should celebrate 25 years of modern NIMV, sing “Happy Birthday”, and make a wish for this therapeutic modality that has contributed so much to the advancement of respiratory medicine over the last 25 years to continue to help advancements in our specialist area for many years to come.
Conflict of InterestsThe authors declare that they have no conflict of interests.
Please cite this article as: Díaz Lobato S, Mayoralas Alises S. La ventilación mecánica no invasiva moderna cumple 25 años. Arch Bronconeumol. 2013;49:475–479.