Asthma, a chronic inflammatory disease of the airways, is associated with airway mucus hypersecretion (AMH) due to mechanisms such as increased mucin secretion and plasma exudation mediated by interleukins (ILs) 4, 9, and 13. These ILs participate in inflammation through metaplasia of goblet cells, which are key drivers of mucociliary dysfunction and biofilm formation.1–6 The current definition of AMH, dating to 1965, refers to patients with chronic obstructive pulmonary disease (COPD) and chronic bronchitis: presence of productive cough lasting more than 3 months for more than 2 consecutive years.7 In those patients, the importance of AMH has led to the development of questionnaires such as the Cough and Sputum Assessment Questionnaire (CASA-Q),8 focused on evaluating cough and sputum descriptively (frequency, severity, etc.) and on determining impact on activities of daily living. However, the CASA-Q was not designed for, nor has it been validated in, patients with asthma, in whom AMH is especially relevant, as not only is it treatable, it is a risk factor associated with greater asthma severity, exacerbations, respiratory functional impairment, mortality, and less responsiveness to corticosteroid treatment.9–12 However, no instrument is as yet available for objective assessment of AMH, which justifies the development and validation of a specific AMH questionnaire for patients with asthma. Our objective was to develop a questionnaire to specifically evaluate AMH in patients with asthma. Our study was approved by the Clinical Research Ethics Committee (Code: IIBSP-TSC-2022-09).
Our Airway Mucus Secretion Test (T-SEC), designed to assess AMH in patients with asthma, was developed in 2 phases, following a design as previously used for other asthma questionnaires and projects, such as the Test of Adherence to Inhalers13 and a set of care quality indicators for severe asthma units.14
In phase 1, an executive committee composed of 8 asthma experts from 5 Spanish hospitals developed an initial draft version with 12 questions, tested on 10 patients with asthma to obtain their opinions. Based on their feedback, certain questions were corrected, modified, or amplified. The results were next presented as part of the Asthma Integrated Research Programme (PII) of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) during its winter meeting in Tarragona (Spain) in February 2022, when pulmonologists and other healthcare providers (such as nurses) with expertise or an interest in asthma were invited to participate in development phase 2. Input from patients and from SEPAR PII participants led to the draft questionnaire being expanded to 15 questions.
Phase 2 consisted of a Delphi survey, with 31 and 29 panellists participating in the first and second round, respectively, using an interactive platform.15 The participating pulmonologists and other healthcare providers originated in 10 different Spanish autonomous regions, and most (77.9%, n=24) had over 10 years’ experience in asthma management (no patients participated in the Delphi survey). The Rand Healthcare Corporation-University of California (Rand/UCLA) methodology16 was used to analyze Delphi panel consensus. This internationally recognized method combines the best available scientific evidence with the collective judgement of experts.
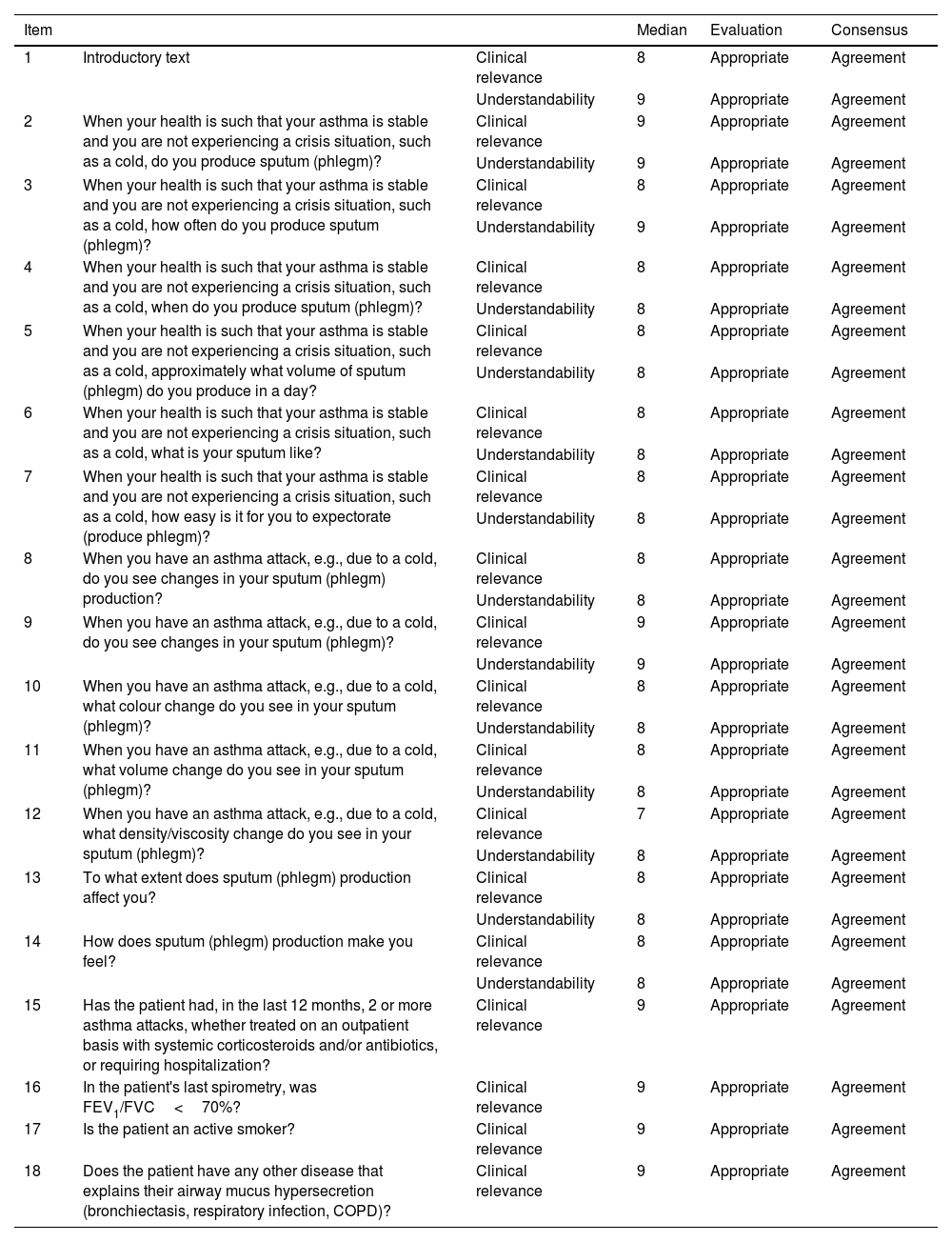
In the first Delphi round, panellists anonymously evaluated 18 items, 2 to evaluate the introductory questions and 16 potential questionnaire items. Clinical relevance and understandability were scored on a 9-point Likert scale (9=total agreement, 1=total disagreement).17 Based on median scores, the items were classified as appropriate (agreement median 7–9), unclear (agreement median 4–6 or any median reflecting disagreement), or inappropriate (agreement median 1–3). Agreement was defined as scoring within the range containing the median by at least two thirds of the panel, disagreement as scoring outside that range by under a third of the panel, and neutrality as neither agreement nor disagreement. All first Delphi round items obtained a median of 7 or >7, reflecting appropriateness and 100% consensus (Table 1A). No item was rated as inappropriate or unclear, and there was no disagreement regarding any item.
Delphi first round. Detailed results for each T-SEC item.
| Item | Median | Evaluation | Consensus | ||
|---|---|---|---|---|---|
| 1 | Introductory text | Clinical relevance | 8 | Appropriate | Agreement |
| Understandability | 9 | Appropriate | Agreement | ||
| 2 | When your health is such that your asthma is stable and you are not experiencing a crisis situation, such as a cold, do you produce sputum (phlegm)? | Clinical relevance | 9 | Appropriate | Agreement |
| Understandability | 9 | Appropriate | Agreement | ||
| 3 | When your health is such that your asthma is stable and you are not experiencing a crisis situation, such as a cold, how often do you produce sputum (phlegm)? | Clinical relevance | 8 | Appropriate | Agreement |
| Understandability | 9 | Appropriate | Agreement | ||
| 4 | When your health is such that your asthma is stable and you are not experiencing a crisis situation, such as a cold, when do you produce sputum (phlegm)? | Clinical relevance | 8 | Appropriate | Agreement |
| Understandability | 8 | Appropriate | Agreement | ||
| 5 | When your health is such that your asthma is stable and you are not experiencing a crisis situation, such as a cold, approximately what volume of sputum (phlegm) do you produce in a day? | Clinical relevance | 8 | Appropriate | Agreement |
| Understandability | 8 | Appropriate | Agreement | ||
| 6 | When your health is such that your asthma is stable and you are not experiencing a crisis situation, such as a cold, what is your sputum like? | Clinical relevance | 8 | Appropriate | Agreement |
| Understandability | 8 | Appropriate | Agreement | ||
| 7 | When your health is such that your asthma is stable and you are not experiencing a crisis situation, such as a cold, how easy is it for you to expectorate (produce phlegm)? | Clinical relevance | 8 | Appropriate | Agreement |
| Understandability | 8 | Appropriate | Agreement | ||
| 8 | When you have an asthma attack, e.g., due to a cold, do you see changes in your sputum (phlegm) production? | Clinical relevance | 8 | Appropriate | Agreement |
| Understandability | 8 | Appropriate | Agreement | ||
| 9 | When you have an asthma attack, e.g., due to a cold, do you see changes in your sputum (phlegm)? | Clinical relevance | 9 | Appropriate | Agreement |
| Understandability | 9 | Appropriate | Agreement | ||
| 10 | When you have an asthma attack, e.g., due to a cold, what colour change do you see in your sputum (phlegm)? | Clinical relevance | 8 | Appropriate | Agreement |
| Understandability | 8 | Appropriate | Agreement | ||
| 11 | When you have an asthma attack, e.g., due to a cold, what volume change do you see in your sputum (phlegm)? | Clinical relevance | 8 | Appropriate | Agreement |
| Understandability | 8 | Appropriate | Agreement | ||
| 12 | When you have an asthma attack, e.g., due to a cold, what density/viscosity change do you see in your sputum (phlegm)? | Clinical relevance | 7 | Appropriate | Agreement |
| Understandability | 8 | Appropriate | Agreement | ||
| 13 | To what extent does sputum (phlegm) production affect you? | Clinical relevance | 8 | Appropriate | Agreement |
| Understandability | 8 | Appropriate | Agreement | ||
| 14 | How does sputum (phlegm) production make you feel? | Clinical relevance | 8 | Appropriate | Agreement |
| Understandability | 8 | Appropriate | Agreement | ||
| 15 | Has the patient had, in the last 12 months, 2 or more asthma attacks, whether treated on an outpatient basis with systemic corticosteroids and/or antibiotics, or requiring hospitalization? | Clinical relevance | 9 | Appropriate | Agreement |
| 16 | In the patient's last spirometry, was FEV1/FVC<70%? | Clinical relevance | 9 | Appropriate | Agreement |
| 17 | Is the patient an active smoker? | Clinical relevance | 9 | Appropriate | Agreement |
| 18 | Does the patient have any other disease that explains their airway mucus hypersecretion (bronchiectasis, respiratory infection, COPD)? | Clinical relevance | 9 | Appropriate | Agreement |
In the second Delphi round, with a view to reaching a consensus on questionnaire structure and wording and reducing the number of questions (to facilitate clinical use), panellists were asked to respond dichotomously to a smaller set of 6 questions (Table 1B). The completed Delphi survey resulted in a T-SEC questionnaire consisting of 6 single-select multiple-choice questions (Fig. 1). Responses are scored from lowest to highest, reflecting least to greatest AMH severity, using a scale to be defined on validating the questionnaire in phase 3.
Delphi second round (dichotomous responses). Detailed results for each T-SEC item.
| Item | Question | Responses | n (%) |
|---|---|---|---|
| 1 | Choose the option that you think patients will best understand between the two proposals for referring to “bronchial hypersecretion” in the T-SEC | A. Mucus (phlegm) production | 10 (34.5%) |
| B. Expectoration (coughing up phlegm) | 19 (65.5%) | ||
| 2 | Choose the wording that you consider most appropriate for the T-SEC | A. When your health is such that your asthma is stable and you are not experiencing a crisis situation, such as a cold, do you produce sputum (phlegm)? | 16 (51.2%) |
| B. When your health is such that your asthma is stable (there is no crisis), do you produce sputum (phlegm)? | 13 (44.8%) | ||
| 3 | Choose the wording that you consider most appropriate for the T-SEC | A. Is the patient an active smoker? | 2 (6.9%) |
| B. Is the patient an active or ex-smoker? | 27 (93.1%) | ||
| 4 | Choose the wording for response “c” that you consider most appropriate for the T-SEC | A. Mid-sized coffee cup (30–125mL) | 9 (31%) |
| B. Espresso-coffee cup or white-coffee cup (30–125mL) | 20 (69%) | ||
| 5 | Choose the question and responses that you consider most appropriate for the T-SEC | A. When you have an asthma attack, e.g., due to a cold, do you see changes in your sputum (phlegm) production? RESPONSES: a. YES b. NO | 10 (34.5%) |
| B. When you have an asthma attack, e.g., due to a cold, do you see changes in your sputum (phlegm) production? RESPONSES: a. YES, I start or continue to produce sputum in a crisis situation b. NO, I don’t produce sputum in a crisis situation. | 19 (65.5%) | ||
| 6 | Choose the question and responses that you consider most appropriate for the T-SEC | A. When you have an asthma attack, e.g., due to a cold, do you see changes in your sputum (phlegm)? RESPONSES a. YES, the colour, volume and/or thickness of the sputum has changed b. NO, the sputum is the same | 16 (51.2%) |
| B. ALL the following statements:i. STATEMENT: When you have an asthma attack, e.g., due to a cold, do you see changes in your sputum (phlegm)? RESPONSES a. YES, the colour, volume and/or thickness of the sputum has changed b. NO, the sputum is the sameii. STATEMENT: When you have an asthma attack, e.g., due to a cold, does the colour of your sputum (phlegm) change? RESPONSES: a. The colour is clearer b. The colour is darker c. There is no changeiii. STATEMENT: When you have an asthma attack, e.g., due to a cold, what volume change do you see in your sputum (phlegm)? RESPONSES a. The volume is less b. The volume is greater c. There is no changeiv. STATEMENT: When you have an asthma attack, e.g., due to a cold, how does the thickness of your sputum (phlegm) change? RESPONSES a. It is less thick b. It is thicker c. There is no change | 13 (44.8%) |
A further 2 exploratory questions were also posed to the experts, regarding the best options for referring to AMH from the patients’ perspective, and the most appropriate time frame for administering the questionnaire. In their first round responses, panellists ranked both “mucus (phlegm) production” and “expectoration (removing phlegm)” highest in terms of referring to AMH (22.6%, n=7), and 3 months as the best time frame (77.4%, n=24). In the second round, the best option to refer to AMH from the patients’ perspective was considered to be “expectoration (coughing up phlegm)” (65.5%, n=19). Finally, the same experts met again to choose the questions that would form part of the T-SEC, based on the Delphi results, Likert scores, and the panellists’ comments.
As far as we are aware, the T-SEC questionnaire, described in this letter, is the first such instrument to evaluate AMH in patients with asthma. Consisting of 7 questions agreed upon by experts in asthma and scored high to low to reflect AMH severity, T-SEC is currently in development phase 3, consisting of its validation through a statistical-psychometric analysis.
An evident limitation of this study is that we advance reporting the questionnaire without the corresponding validation study, currently underway. However, given the growing interest in understanding AMH in asthma, and particularly severe asthma, it seemed important not to delay unduly. Another limitation is the lack of a gold standard to measure AMH, and hence, the quantity of phlegm (mL and/or grams) produced in 24h18 is calculated and scored according to a scale established by lung CT.19,20 Another limitation of the study is that only Spanish professionals participated, given that the study is part of a national project.
The interest of this letter is the lack of a specific instrument to assess AMH in patients with asthma. T-SEC is the first such instrument developed for this purpose. The fact that AMH in asthma is treatable makes it important to have a reliable, objective, and specific means of assessing asthma that can easily and rapidly be used in routine clinical practice.
In conclusion, in T-SEC we describe the first questionnaire specifically developed to evaluate AMH in patients with asthma, part of a project undertaken by a group of SEPAR asthma experts. T-SEC is composed of 7 single-select multiple-choice questions, scored high to low to reflect AMH severity. Its administration in clinical practice will facilitate rapid and easy AMH detection in patients with asthma.
FundingThis research was supported by a grant from the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR).
Conflicts of interestsEP has received conference travel and attendance expenses from Gebro Pharma, Chiesi, FAES Farma, GlaxoSmithKline, AstraZeneca, and Sanofi, and has received fees for talks at meetings sponsored by GlaxoSmithKline, and funds/grants for research projects from state agencies and non-profit foundations.
JLGR has received speaker's fees from GSK, Astrazeneca, Teva, Chiesi, Zambon, Grifols, and Sanofi, and consulting fees from GSK, Astrazeneca, and Grifols.
CC has received financial aid in the last 3 years from Astra Zeneca, Chiesi, Novartis, Sanofi, GSK, and Gebro Pharma for advisory services, papers, research studies, attendance at congresses, and training courses.
CAS in the last 3 years has received fees for speaking at sponsored meetings from Astrazeneca, Boehringer-Ingelheim, Chiesi, Gebro, GSK, and Sanofi, has received assistance with travel to meetings from Astrazeneca and Chiesi, and has acted as a consultant for Astrazeneca, Chiesi, GSK, and Zambon.
LPL reports grants, personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from GSK, grants, personal fees and non-financial support from TEVA, personal fees and non-financial support from Chiesi, grants, personal fees and non-financial support from Sanofi, personal fees from MSD, personal fees from TECHDOW PHARMA, grants, personal fees and non-financial support from FAES, personal fees from Leo-Pharma, grants and personal fees from GEBRO, personal fees from GILEAD, outside the submitted work.
VP, in the last 3 years, has received speaker fees for meetings sponsored by Astrazeneca, Boehringer-Ingelheim, Chiesi, Gebro, GSK, Luminova-Medwell, and Sanofi, has received assistance to travel to meetings from Astrazeneca and Chiesi, and has acted as a consultant for Astrazeneca, Chiesi, GSK, and Menarini.
ACL has received fees in the last 3 years for talks at meetings sponsored by AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Ferrer, GlaxoSmithKline, MSD, Novartis, Orion Pharma, Zambón, and Sanofi, has received travel and attendance expenses for conferences from Bial, Gebro, GlaxoSmithKline, Sanofi, Novartis, and TEVA, and has received funds/grants for research projects from several state agencies, non-profit foundations, AstraZeneca, and GlaxoSmithKline.
Grateful thanks to Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) Asthma Integrated Research Programme (PII) participants in the Delphi survey: Alicia Padilla Galo, Ana Lapuente Torrents, Ana María Stok, Ana Pueyo, Anabel Garcia Onieva, Andrea Trisán, Beatriz Arias Arcos, Carlos Martínez Rivera, Celia Pinedo, Concepción Morales García, Elena Curto Sanchez, Elena Martín, Eva Martínez Moragón, Fernando Sánchez-Toril López, Ignacio Lobato Astiárrago, Inmaculada Plasencia García, Irene de Lorenzo, Javier Hernández González, Laura Anoro Abenoza, Lourdes Lázaro Asegurado, Maria Jose Espinosa, Mariana Muñoz Esquerre, Marina Blanco, Núria Marina Malanda, Rocío Magdalena Díaz Campos, Sylvia Sánchez Cuellar, Teresa Bigorra.