Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) nowadays represents the standard for the diagnosis and staging of mediastinal lymph nodes and masses in most hospital centers. In comparison to the former gold standard of mediastinoscopy, complication rate is lower yielding a similar diagnostic accuracy.1
We report a case of septic mediastinitis following an EBUS-TBNA of a mass in the posterior mediastinum, later diagnosed as follicular dendritic cell sarcoma.
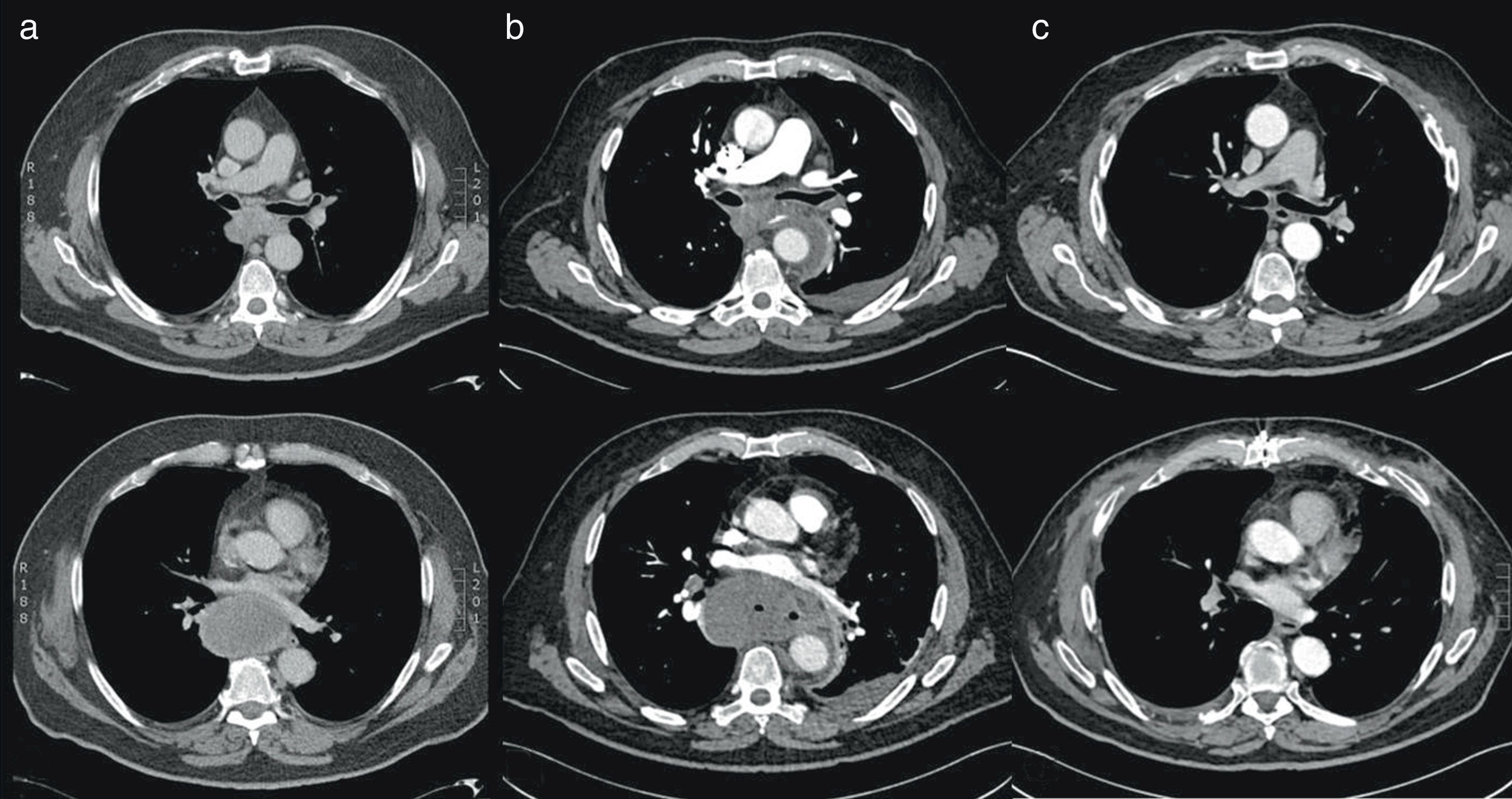
A 71-year-old man was referred to our hospital to perform EBUS-TBNA of a newly diagnosed tumor located in the posterior mediastinum. The patient was a non-smoker and had always been healthy except for an essential hypertension diagnosed 15 years ago. Because of a persistent cough a chest CT had been performed revealing a tumor of the posterior mediastinum of 74×71×53mm size adjacent to the esophagus and compressing the left ventricle (Fig. 1a). EBUS-TBNA showed a subcarinal tumor with a short-axis diameter of approximately 5cm. Sonographic images suggested an isoechogenic structure without necrotic lesions which was then punctured 4 times using a 22G Olympus needle. There was no bleeding or any other complications related to the procedure and the patient was discharged home the following day without antibiotic treatment as according to our guidelines. The pathology report described a mitotically active solid tumor, a further differentiation could not be made. 14 days after EBUS-TBNA the patient was reevaluated because of the new onset of fever, dyspnea and progressive confusion. He presented himself disorientated with acute respiratory distress (breathing rate 42/min, oxygen saturation of 89% without oxygen supply). Temperature was 38.8°C, heart rate 110/min and blood pressure within normal limits. Blood tests revealed a WBC of 22.12×109/l and a C-reactive protein level of 307mg/l (normal, <0.5mg/l). Chest CT described new gas inclusions in the previously punctured tumor and a periaortic fluid collection of the descending aorta (Fig. 1b). An acute mediastinitis leading to severe sepsis, moderate ARDS, septic encephalopathy and prerenal failure was diagnosed. The patient was transferred to the intensive care unit; an antibiotic treatment with Piperacillin–Tazobactam was started, following emergency resection of the tumor in toto via clamshell incision. The tumor was perforated into the mediastinum and therefore debridement plus lavage with temporary retrocardial vacuum sealing was necessary. After repeated scheduled debridement and VAC change during 48h after resection, the chest was closed definitely on the 5th day. The patient was extubated the same day and discharged to the ward at day 15. The presence of Parvimonas micra was detected in one blood culture, periaortic tissue and pleural biopsy. The patient had a favorable clinical and biochemical outcome and he was discharged to rehabilitation one month later. The pathology report described a necrotic tumor expressing only Synaptophysin, CD21 and CD23, suggesting the diagnosis of a follicular dendritic cell sarcoma, although Synaptophysin is not commonly expressed in FDC-Sarcomas. A follow-up Chest-CT 3 months following tumor resection showed no tumor-relapse (Fig. 1c).
Sections of an axial chest CT of the mediastinal presenting the tumor in the posterior mediastinum. (a) Tumor of the posterior mediastinum before EBUS measuring 74×71×53m. (b) Tumor of 5.4cm (short axis) 14 days after EBUS with puncture. (c) Mediastinal axis 3 months following resection of the tumor.
EBUS-TBNA has become the new standard procedure for the diagnosis of mediastinal lesions accessible via the major pathway.1 Its advantages over mediastinoscopy are undisputable, being minimally invasive, safe and performable in moderate sedation but still achieving a high diagnostic yield of 88%–96% compared to 80% in mediastinoscopy.2–5 The complication rate of EBUS-TBNA varies between 0.07% and 1.44%.4,5 Possible risk factors for complications and an escalation of care are age >70 years, inpatient status and undergoing deep sedation or general anesthesia.6 Infection is caused by inoculation of oral pathogens into the mediastinal tumor when applying the aspiration needle to gain tissue samples. Possible complications include hemorrhage, pneumothorax and mainly infectious complications like mediastinitis and pericarditis.7
In this case mediastinal infection resulted as a complication of EBUS-TBNA of a tumor of unknown origin. Since the culprit pathogen is supposed to be introduced by the aspiration needle, we found consistent with this theory P. micra in periaortic tissue, pleural biopsy and one blood culture. P. micra is an anaerobic, Gram positive coccus normally found in oral and gastrointestinal flora.8
Given that bacteremia may result from bacterial mucosal penetration above the vocal cords, using a mouth rinse or performing full mouth disinfection before procedure might reduce the risk of infection.9 According to our guidelines we do not use antibiotic prophylaxis in immunocompetent patients compared to other clinics giving a single injection of an antibiotic.10 To date there is no study questioning the use of antiseptic mouth wash or prophylactic antibiotics including its optimal duration of treatment and the number needed to treat to prevent an infection following EBUS-TBNA. One approach could be to administer antibiotics (and mouth rinse) only to high risk patients (as we do already in the case of lung transplant patients), such as immunocompromised patients due to diabetes or medical treatment.11
Whether or not a mediastinal lesion should have diagnostic approach before resection is debatable. Whenever a radically resection of a mediastinal mass is possible, there is no need for further diagnostic tissue work up. Furthermore, as shown in this case, invasive diagnostic can not only delay an operation but may trigger complications. However if pathological findings influence treatment (neoadjuvant therapy) or surgical approach, interventional diagnostics is justified.
In conclusion, we presented a patient who developed an acute mediastinitis with severe sepsis, moderate ARDS, septic encephalopathy and prerenal failure after EBUS-TBNA of a follicular dendritic cell sarcoma. EBUS-TBNA is a very safe, reliable and commonly established technique yielding a high diagnostic rate but with its widespread use we will have to face new questions of how to deal with possible serious complications and even more important how to prevent them.