The patient is a 21-year-old female, diagnosed with cryptogenic cirrhosis at the age of 9. She presented with left post-pneumonic empyema that did not remit with conventional medical management and evolved with fistulization to the skin in the 7th intercostal space in the left subscapular region. We performed an open thoracic window procedure, and on the 6th day the patient was sent home with a portable vacuum-assisted closure device, with changes of the material every 4 days until the cavity was completed obliterated (92 days). Imaging tests showed full expansion of the lung, and chest wall reconstruction was performed with titanium rods. The high mortality of empyema in patients with liver disease requires both implementing and searching for new adjuvant therapies, like the use of vacuum-assisted closure systems and reconstruction with titanium rods. Controlled studies with a wide range of cases are needed for proper evaluation.
Mujer de 21 años diagnosticada de cirrosis criptogénica desde los 9 años de edad, que presentó empiema izquierdo posneumónico que no remitió con el tratamiento médico convencional y evolucionó con fistulización hacia la piel en el séptimo espacio intercostal a nivel subescapular izquierdo. Se realizó una ventana torácica abierta y al sexto día se envió a su domicilio con colocación de sistema cerrado de succión portátil, con cambios cada 4días del material hasta la obliteración total de la cavidad (92 días). Se observó por imagen una expansión completa del pulmón y se realizó reconstrucción de la pared torácica con barras de titanio. La alta mortalidad del empiema, en los pacientes con hepatopatías, requiere la implementación y la búsqueda de nuevas terapias adyuvantes, como la utilización del sistema cerrado de succión y la reconstrucción con barras de titanio. Para una adecuada evaluación, se requieren estudios controlados con una serie de casos amplia.
Intrathoracic use of a vacuum-assisted closure device (Vacuum Assisted Closure™, VAC™) is a novel technique, with growing evidence, obtained mainly in patients with empyema following lung resection, to suggest that its use as adjuvant treatment in patients with open thoracic window due to recurrent or persistent empyema can reduce the morbidity and hospital stay.1
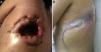
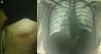
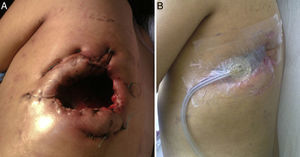
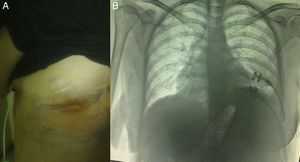
Clinical ObservationThe patient was a 21-year-old female, diagnosed with cryptogenic cirrhosis at the age of 9, with portal hypertension syndrome and oesophageal varices. Four months before attending our department, she was diagnosed with left post-pneumonic empyema that did not remit with antimicrobial therapy. Thoracentesis and insertion of a chest drain was performed on two occasions, and evolved with infiltration of the wall and fistulization to the skin in the seventh intercostal space in the left subscapular region. She was admitted with deterioration in her general condition and pleuritic chest pain in the left hemithorax. Physical examination revealed symptoms of pleural effusion in the lower half of the left hemithorax, with heart rate 98 beats/min, respiratory rate 30 breaths/min, blood pressure 90/60mmHg and temperature 38.2°C; laboratory test results showed leukocytes 15900/mm3. The patient was classified as Child–Pugh class B liver disease. Staphylococcus haemolyticus was found in the empyema culture. Imaging tests (chest radiograph and computed tomography) showed a posterior-basal effusion image occupying 60% of the pleural cavity of the left hemithorax, a collapsed lung and pachypleuritis. It was decided to perform an open thoracic window procedure (Fig. 1), resecting 9cm in length from the posterolateral region of costal arches 8 and 9. Abundant purulent material and thickened visceral and parietal pleura approximately 1cm thick were found during the procedure, but decortication was not performed. Osteomyelitis of the eighth costal arch and a fistulous tract in the seventh intercostal space were also observed. The procedure also consisted of packing the pleural cavity with dressings for 48h, based on thromboelastography findings of prolonged reaction (R) and coagulation (K) times. The cavity was then re-examined and repacked with dressings for a further 48h. An intrathoracic (IT) VAC™ system was applied on the fourth day and on the sixth day the patient was discharged home with a portable VAC™ system; we used an initial suction pressure of 75mmHg and intensity of 30mmHg for 2 days before increasing the suction to 125mmHg and intensity to 40mmHg. There was a gradual reduction in exudate volume, from an average of 300ml per day during the first 2 weeks to an average of 300ml every 4 days (after six weeks treatment). The sponge (VAC Granufoam SilverMR Large Dressing) was changed every 4 days, observing a clear improvement after 15 days of use. An initial residual cavity of 18cm×9cm×9cm was found, reduced to 8cm×2cm×2cm after six weeks use, so it was decided to continue with VAC™-IT for a further six weeks until complete obliteration of the cavity (92 days). The radiographic image showed complete lung expansion. During the course of treatment, serial cultures were performed by biopsy after one and two months, showing growth of Staphylococcus haemolyticus and Staphylococcus hominis. The outpatient antibiotic regimen was therefore completed, with the final culture negative after three months. The patient was scheduled for chest wall reconstruction with titanium rods (STRATOS™ system), which was performed with no complications. She progressed satisfactorily and was discharged one week after the procedure. Preoperative lung function tests in our patient showed an FVC of 54% of the predicted and an FEV1 of 50% of the predicted; 4 months after chest wall construction, the FVC was 87% of the predicted and the FEV1 was 92% of the predicted. Follow-up tests in outpatients up to 8 months later did not reveal any complications (Fig. 2).
Liver cirrhosis is considered a contraindication for lavage and decortication, due to the frequent haemostatic and nutritional abnormalities that significantly increase morbidity and mortality.2–5 Chen et al. recorded a mortality of 31.5%–48.4% when these patients were treated with thoracentesis or drainage catheters, and 21.1% when treated with thoracoscopy, with no significant difference in the mortality when thoracoscopic vs non-thoracoscopic treatment was applied.5 In 2006, Varker and Ng6 first described the successful use of intrathoracic VAC™ (VAC™-IT) in a post-lobectomy empyema. Since then, single cases and case series using VAC™-IT have been reported, mainly in post-lobectomy or post-pneumonectomy empyema, suggesting it as an adjuvant treatment for the management of empyemas in patients with complications, as it decreases the length of the hospital stay and potentially reduces morbidity.1,6–14 We did not find reports on the use of VAC™ in open thoracic window procedures performed due to empyema in patients with liver cirrhosis.
The use of VAC™-IT in our case meant early hospital discharge and outpatient follow-up; neither analgesia nor sedation was required during the system sponge change, and the patient always reported tolerable pain. In comparing our results with the studies by Palmen el al.12 and Saadi et al.,13 which recorded a duration of VAC™ therapy of 39±17 days (range 6–66 days), it should be stressed that these authors did not describe the size of the residual cavity nor the grade of pachypleuritis. In this respect, we recorded the size of the thoracic window performed in our patient (9cm×6.5cm in diameter), an intrathoracic residual pleural space of 21cm×9cm×9cm, a pleural rind of approximately 1cm on average and in which decortication was not carried out, and we also decided to use VAC™-IT until obliteration of the residual cavity and complete lung expansion, factors that contributed to the total therapy time with the vacuum-assisted closure system. It is important to note that Rocco et al.15 reported 2 cases in which they had to discontinue the vacuum-assisted closure system permanently due to acute chest pain and hypotension, and they described one case in which the sponge used adhered to the granulation tissue and could only be removed under thoracoscopy.
Chest wall reconstruction following thoracic window surgery has been reported mainly using muscle flaps (myoplasty) and thoracomyoplasty.16–19 Having observed adequate lung expansion in our patient following the use of VAC™-IT, we decided to perform chest wall reconstruction with rib restoration using titanium plates and clips (STRATOS™ system), which we believe is a more physiological restoration of the wall. We did not find any previous reports of a reconstruction using this system in a similar case.
ConclusionThe high mortality of empyema in patients with liver disease requires searching for and implementing new adjuvant therapies, such as the use of VAC™-IT and possible reconstruction with titanium rods. Controlled studies with extensive case series are required for proper evaluation.
Conflict of InterestsThe authors declare no conflicts of interests with respect to the present article.
Please cite this article as: Munguía-Canales DA, et al. Manejo del empiema con un sistema cerrado de succión y reconstrucción de ventana torácica en un paciente con cirrosis hepática. Arch Bronconeumol. 2013;49:447–9.