About 20% of patients infected by the SARS-CoV-2 virus develop Coronavirus Disease 2019 (COVID-19) pneumonia and require hospitalization.1 Some recent reports have shown that some of them may present lung function abnormalities at discharge,2,3 or soon afterwards.4–6 Here, we: (1) describe the presence and characteristics of lung function abnormalities 3 months after hospital discharge in a large prospective cohort of well characterized patients hospitalized because of COVID-19 in our institution; and, (2) explore potential clinical predictors these short-term lung function sequelae.
We included in the study 172 patients discharged from Hospital Clinic in Barcelona because of COVID-19 pneumonia from 4th of March to 27th April 2020. The study protocol was approved by our Ethical Review Board (HCB/2020/0422), and all patients signed their informed consent. Demographic, clinical and biological characteristics were recorded on hospital admission and 3 months after discharge. All patients followed the current Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) consensus for post-COVID-19 clinical follow-up.7 The severity of COVID-19 was classified as moderate in patients who did not require supplemental oxygen and severe in those who did, according to WHO recommendations.8 Spirometry and carbon monoxide lung diffusion capacity (DLCO) were measured (Medisoft, Sorinnes, Belgium) following international recommendations9,10 3 months after hospital discharge adapted to current pandemic situation.11,12
Mean age was 56.1±19.8 years-old and 57% of patients were males. Hypertension (37%) and diabetes (16%) were frequent prior comorbid conditions. Most patients (70%) had severe COVID-19 and 43% were admitted to the intensive care unit (ICU). Length of stay in ICU was 14.6±27.3 days and in hospital 20.1±16.3 days. On average, 3 months (101.5±19.9 days) after discharge spirometry was normal (median [interquartile range] FEV1 94 [80–105]%, FVC 90 [80–100]% of predicted and FEV1/FVC ratio 0.80 [0.75–0.84]) but DLCO was slightly reduced (77 [64–88]% of predicted). Yet, a more granular analysis showed that 109 patients (63%) had evidence of altered pulmonary function at 3 months of follow-up, as defined by values of FEV1, FVC and/or DLCO<80% of reference. The most frequent abnormality was reduced DLCO (98 patients (57%)), followed by low FEV1 (43 patients (25%)) and low FVC (42 patients (24%)).
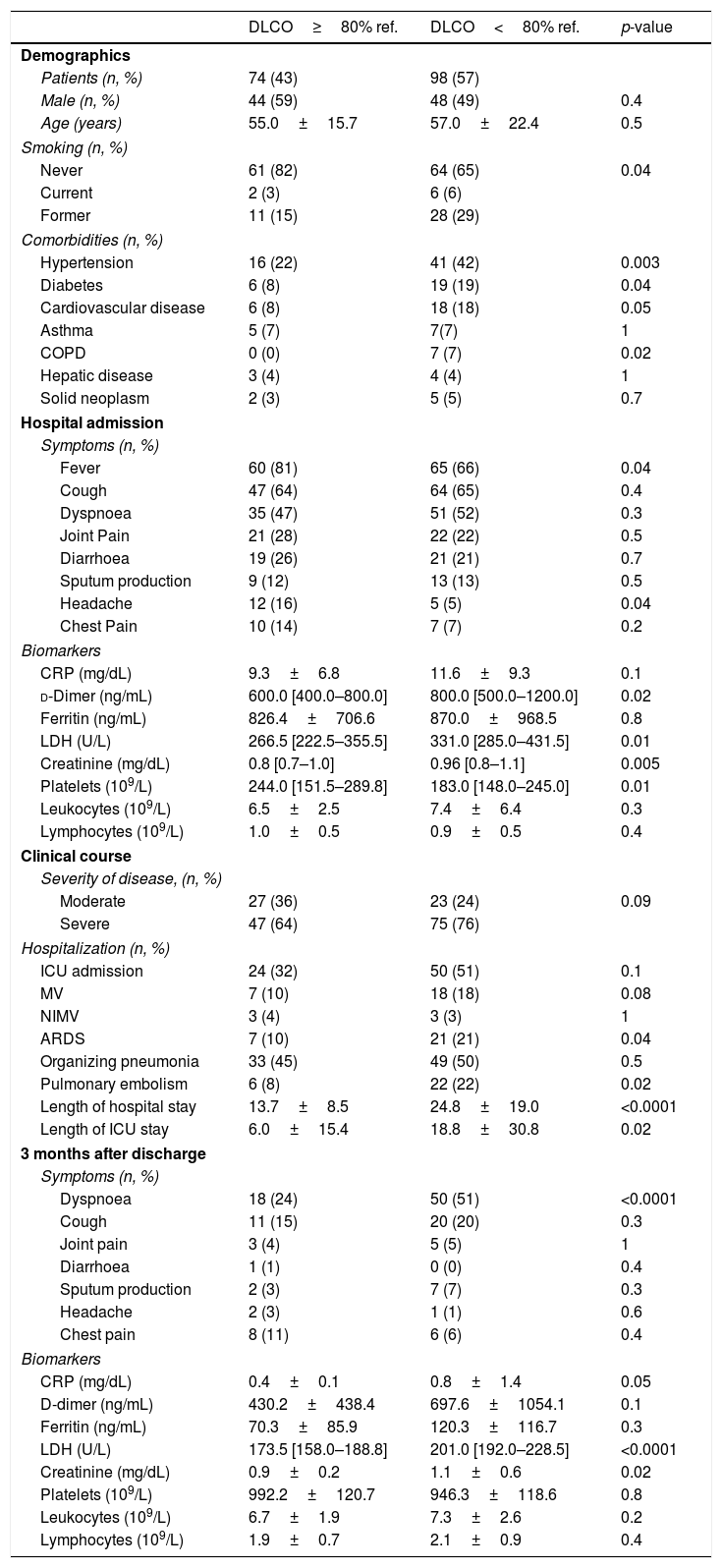
Table 1 compares the main characteristics of patients with normal and abnormal DLCO values 3 months after discharge. We observed that: (1) the later included more smokers and patients with hypertension, diabetes, cardiovascular disease or chronic obstructive pulmonary disease (COPD); (2) on admission, these patients showed higher levels of d-dimer, lactate dehydrogenase (LDH), and creatinine (and reduced platelet counts); (3) during hospitalization, differences in WHO severity of disease failed to reach statistical significance, but the prevalence of acute respiratory distress syndrome (ARDS) and pulmonary embolism was higher in patients with reduced DLCO after discharge. These patients also showed prolonged ICU and hospital stay; and, finally, (4) 3 months after discharge, patients with reduced DLCO were more dyspnoeic and showed higher values of C-reactive protein (CRP), LDH and creatinine (Table 1).
Comparison of COVID-19 patients with and without abnormal DLCO at 3 months. Data is presented as mean±standard deviation or median [interquartile range].
| DLCO≥80% ref. | DLCO<80% ref. | p-value | |
|---|---|---|---|
| Demographics | |||
| Patients (n, %) | 74 (43) | 98 (57) | |
| Male (n, %) | 44 (59) | 48 (49) | 0.4 |
| Age (years) | 55.0±15.7 | 57.0±22.4 | 0.5 |
| Smoking (n, %) | |||
| Never | 61 (82) | 64 (65) | 0.04 |
| Current | 2 (3) | 6 (6) | |
| Former | 11 (15) | 28 (29) | |
| Comorbidities (n, %) | |||
| Hypertension | 16 (22) | 41 (42) | 0.003 |
| Diabetes | 6 (8) | 19 (19) | 0.04 |
| Cardiovascular disease | 6 (8) | 18 (18) | 0.05 |
| Asthma | 5 (7) | 7(7) | 1 |
| COPD | 0 (0) | 7 (7) | 0.02 |
| Hepatic disease | 3 (4) | 4 (4) | 1 |
| Solid neoplasm | 2 (3) | 5 (5) | 0.7 |
| Hospital admission | |||
| Symptoms (n, %) | |||
| Fever | 60 (81) | 65 (66) | 0.04 |
| Cough | 47 (64) | 64 (65) | 0.4 |
| Dyspnoea | 35 (47) | 51 (52) | 0.3 |
| Joint Pain | 21 (28) | 22 (22) | 0.5 |
| Diarrhoea | 19 (26) | 21 (21) | 0.7 |
| Sputum production | 9 (12) | 13 (13) | 0.5 |
| Headache | 12 (16) | 5 (5) | 0.04 |
| Chest Pain | 10 (14) | 7 (7) | 0.2 |
| Biomarkers | |||
| CRP (mg/dL) | 9.3±6.8 | 11.6±9.3 | 0.1 |
| d-Dimer (ng/mL) | 600.0 [400.0–800.0] | 800.0 [500.0–1200.0] | 0.02 |
| Ferritin (ng/mL) | 826.4±706.6 | 870.0±968.5 | 0.8 |
| LDH (U/L) | 266.5 [222.5–355.5] | 331.0 [285.0–431.5] | 0.01 |
| Creatinine (mg/dL) | 0.8 [0.7–1.0] | 0.96 [0.8–1.1] | 0.005 |
| Platelets (109/L) | 244.0 [151.5–289.8] | 183.0 [148.0–245.0] | 0.01 |
| Leukocytes (109/L) | 6.5±2.5 | 7.4±6.4 | 0.3 |
| Lymphocytes (109/L) | 1.0±0.5 | 0.9±0.5 | 0.4 |
| Clinical course | |||
| Severity of disease, (n, %) | |||
| Moderate | 27 (36) | 23 (24) | 0.09 |
| Severe | 47 (64) | 75 (76) | |
| Hospitalization (n, %) | |||
| ICU admission | 24 (32) | 50 (51) | 0.1 |
| MV | 7 (10) | 18 (18) | 0.08 |
| NIMV | 3 (4) | 3 (3) | 1 |
| ARDS | 7 (10) | 21 (21) | 0.04 |
| Organizing pneumonia | 33 (45) | 49 (50) | 0.5 |
| Pulmonary embolism | 6 (8) | 22 (22) | 0.02 |
| Length of hospital stay | 13.7±8.5 | 24.8±19.0 | <0.0001 |
| Length of ICU stay | 6.0±15.4 | 18.8±30.8 | 0.02 |
| 3 months after discharge | |||
| Symptoms (n, %) | |||
| Dyspnoea | 18 (24) | 50 (51) | <0.0001 |
| Cough | 11 (15) | 20 (20) | 0.3 |
| Joint pain | 3 (4) | 5 (5) | 1 |
| Diarrhoea | 1 (1) | 0 (0) | 0.4 |
| Sputum production | 2 (3) | 7 (7) | 0.3 |
| Headache | 2 (3) | 1 (1) | 0.6 |
| Chest pain | 8 (11) | 6 (6) | 0.4 |
| Biomarkers | |||
| CRP (mg/dL) | 0.4±0.1 | 0.8±1.4 | 0.05 |
| D-dimer (ng/mL) | 430.2±438.4 | 697.6±1054.1 | 0.1 |
| Ferritin (ng/mL) | 70.3±85.9 | 120.3±116.7 | 0.3 |
| LDH (U/L) | 173.5 [158.0–188.8] | 201.0 [192.0–228.5] | <0.0001 |
| Creatinine (mg/dL) | 0.9±0.2 | 1.1±0.6 | 0.02 |
| Platelets (109/L) | 992.2±120.7 | 946.3±118.6 | 0.8 |
| Leukocytes (109/L) | 6.7±1.9 | 7.3±2.6 | 0.2 |
| Lymphocytes (109/L) | 1.9±0.7 | 2.1±0.9 | 0.4 |
ARDS, acute respiratory distress syndrome; COPD, chronic obstructive pulmonary disease; ICU, Intensive Care Unit; LDH, lactate dehydrogenase; CRP, protein C reactive; MV, mechanical ventilation; NIMV, noninvasive mechanical ventilation.
In relation to the observed spirometric abnormalities 3 months after discharge, we observed that those with reduced FEV1 were more frequently males (76.9% vs 51.2%, p=0.005) with a prior history of cardiovascular disease (34.2% vs 9.4%, p=0.001) and diabetes (28.9% vs 12%, p=0.02). Similarly, those with reduced FVC were also more frequently males (76.3% vs 51.6%, p=0.008) with prior cardiovascular disease (29.7% vs 11.0%, p=0.009). No other differential clinical or biologic differences were observed in patients with or without altered spirometry 3 months after discharge.
These results confirm that survivors of COVID-19 may suffer lung function sequalae. Previous studies showed reduced DLCO in 25% of the patients 3 months after hospital discharge and spirometric alterations in around 10% of them.4,6 In our series, lung function abnormalities were more prevalent, reduced DLCO being observed in 57% of patients and spirometric alterations in about one quarter of them. These differences may be related to the fact that our cohort include more severe patients than those observed in previous studies (43% admitted to ICU compared to 14%6 and 12%4).
In our cohort, a number of clinical characteristics during the acute phase of COVID-19 were associated with reduced DLCO 3 months after discharge, including being a smoker, suffering chronic respiratory, cardiovascular or metabolic comorbidities, having a more severe in-hospital course and presenting higher levels of several biomarkers associated with severe COVID-19, some of which persisted elevated at follow-up; further, patients with reduced DLCO at follow-up were dyspnoeic more often (Table 1).13,14 All in all, these observations contribute to identify, during the acute phase of the disease, a group of COVID-19 patients who deserve monitoring after discharge.
Our study has several limitations. First, the lack of pulmonary function test results before COVID-19 limits any comparison with measurements after discharge, so we do not know if the observed abnormalities were already present before being infected by SARS-CoV-2. For instance, it is not possible to know if the association between smoking and decreased DLCO represented the effect of pre-COVID-19 smoking, COVID-19 or a combination of both. Yet, the proportion of individuals with known COPD before COVID-19 was very small (Table 1). Second, did not study the potential association of functional and structural abnormalities using imaging techniques, as other studies have reported very recently.5,6 Finally, we lack a control group of patients with non-COVID-19 pneumonia.
In conclusion, these results confirm that lung function sequelae, particular reduced DLCO, are frequent 3 months after hospital discharge in COVID-19 patients and illustrate that persistent dyspnoea is a common clinical marker. Besides, our study shows that a number of risk factors can be identified early during the course of the disease.
FundingThe present study has been founded by a research grant from Menarini.
Conflict of interestNone declared.