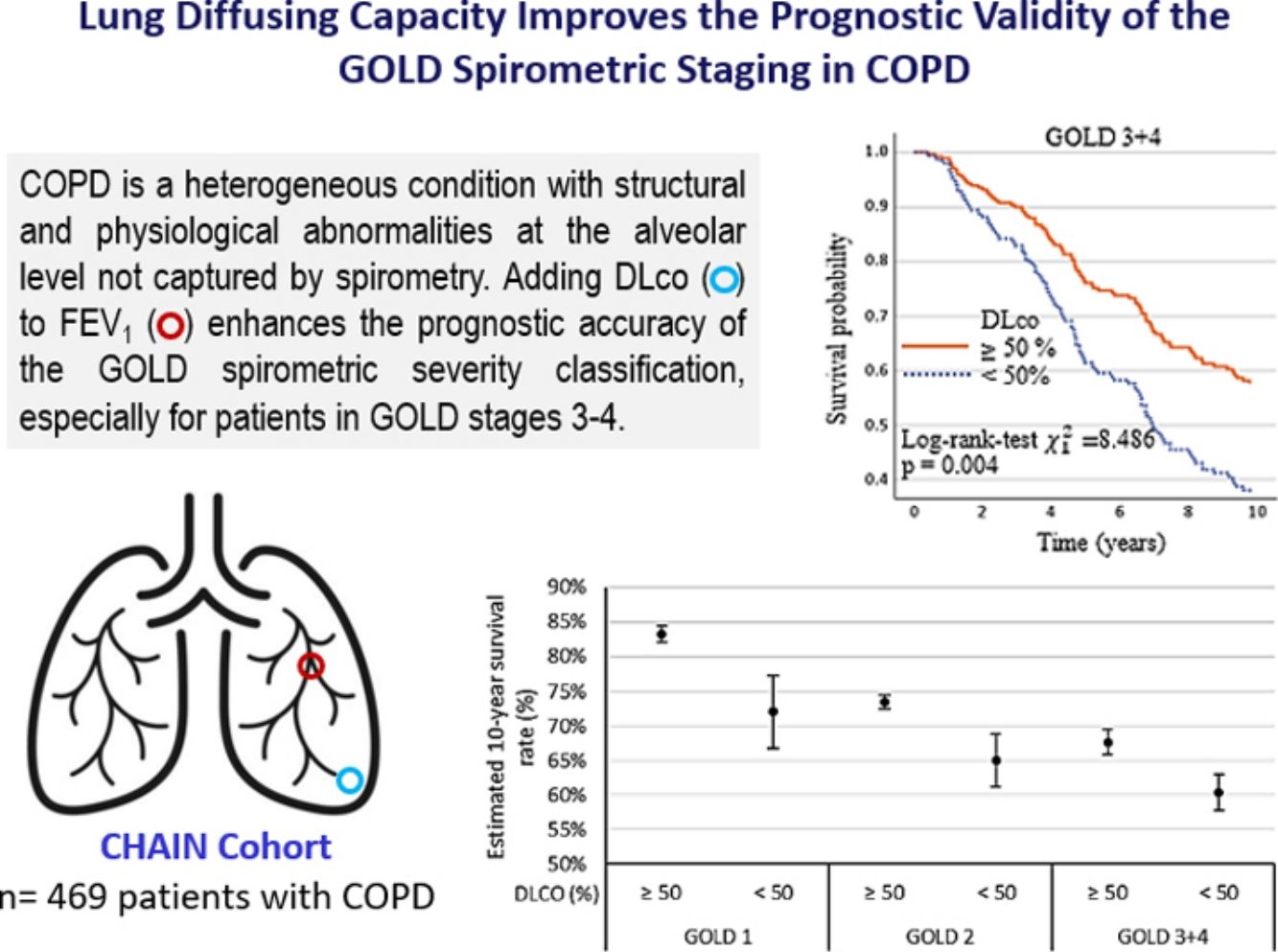
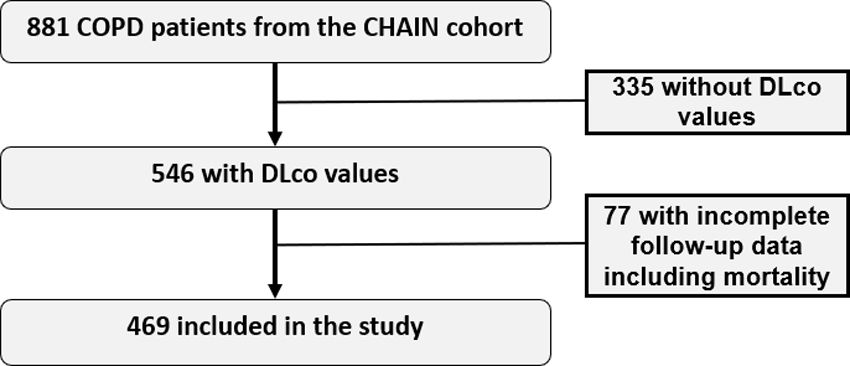
The lung diffusing capacity for carbon monoxide (DLco), a metric of gas transfer, provides physiological information distinct from spirometry. While DLco independently predicts mortality in COPD, its integration into the GOLD spirometric staging (% FEV1) to improve risk assessment, remains unexplored.
ObjectivesTo determine if DLco enhances the predictive power of GOLD spirometric classification for all-cause and respiratory mortality.
MethodsWe followed 469 patients (mean age 64 years, 58% FEV1) with complete lung function tests in the Spanish multicenter CHAIN study for up to 10 years, with mortality as the main outcome. Patients were dichotomized based on DLco impairment (<50% cutoff). A Cox proportional hazard model evaluated the added value of DLco to GOLD FEV1 spirometric staging for all-cause and respiratory mortality. Validation of the results was conducted in the Kingston COPD Canadian cohort (N=300 patients).
ResultsOver time, 184 (39.2%) patients died, 84 (17.9%) from respiratory causes. Adjusted analyses showed DLco<50% independently predicted all-cause [HR=1.83 (95%CI 1.32–2.54, p<0.001)] and respiratory [HR=2.27 (95%CI 1.43–3.60, p<0.001)] mortality. Incorporating DLco<50% increased mortality risk compared to FEV1 alone, particularly in GOLD stages 3 and 4, where survival time decreased by 1.23 years (p=0.002) and 1.25 years (p=0.004) for all-cause and respiratory deaths, respectively. These findings were validated in the Canadian cohort.
ConclusionsAdding DLco to FEV1 enhances the prognostic accuracy of the GOLD spirometric severity classification, especially for patients in GOLD stages 3–4 at higher risk of adverse outcomes.
ClinicalTrials.gov Identifier: NCT01122758.
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide and a major public health issue.1 Disease severity is typically gauged based on the severity of lung function impairment as inferred from decrements in the forced expired volume in the 1st second (FEV1% predicted).2 Given its simplicity, reproducibility, and prognostic ability, the Global Initiative for Obstructive Lung Diseases (GOLD)1,2 has endorsed FEV1% as the key functional predictor of disease severity in COPD. However, recent evidence shows relevant physiological abnormalities with prognostic implications not captured by spirometry.3 For instance, FEV1 is, by definition, largely oblivious to derangements in gas transfer from atmospheric air to capillary blood,1 the fundamental task of the lungs.
The lung diffusion capacity for carbon monoxide (DLco) is a widely available, well-standardized pulmonary function test4 that is influenced by the multiple interconnected mechanisms involved in gas transfer. For instance, DLco is reduced due to abnormalities in ventilation and/or perfusion distribution and the integrity of the alveolar-capillary membrane.5 Since each of these steps is variably affected as COPD progresses toward disablement and death, it is not surprising that a low DLco has been associated with several patient-centered outcomes, e.g., decreased exercise capacity,6,7 worse health status,8 increased risk of exacerbations,9,10 and pulmonary complications after surgical lung resection.11 DLco also strongly predicts mortality, independent of the severity of airflow obstruction.12–14 Therefore, there is a sound rationale for DLco to be considered, in addition to the traditional FEV1, in the GOLD classification of disease severity and risk of a negative outcome.
In this context, the present study was designed to test the hypothesis that DLco% would add to GOLD's FEV1% in predicting respiratory and all-cause mortality across the spectrum of COPD severity. We analyzed 10 years of prospectively collected data from the multicenter COPD History Assessment In SpaiN cohort (CHAIN) to reach this goal. We then tested the reproducibility of these results in a COPD Kingston Canadian cohort.
MethodsSubjects Recruited into the StudyCHAIN is an ongoing observational study of COPD patients recruited between January 2010 and December 2023 at 24 university hospitals in Spain.15 Data were obtained at baseline and repeated annually over ten years. COPD was defined by a smoking history ≥10 pack-years and a post-bronchodilator FEV1/FVC<0.7 after 400μg of albuterol. Patients were stable for at least 6 weeks and received guideline-directed therapy.1 Patients recruited in the Kingston (Canada) cohort between 1995 and 2014 followed a similar recruitment process, also an observational with a yearly follow-up protocol.13 Exclusion criteria were alpha-1-antitrypsin deficiency or uncontrolled comorbidities, including malignancy or other confounding diseases that could interfere with the study. Patient data were anonymized with hierarchical access control to guarantee that information was secured. All participants signed the informed consent. This study has obtained ethics approval from the Comité de Etica de Investigación, Hospital Universitario la Candelaria, Tenerife, review board [IRB n°: 258/2009].
Clinical and Physiological MeasurementsThe methods of the CHAIN study have been published previously.15 In summary, trained staff recorded age, gender, and body mass index (BMI) at all visits. Smoking was determined by history and current status by co-oximetry (piCO Smokerlyzer; Bedfont Scientific). Pulmonary function tests were performed following ATS guidelines.16 Diffusion capacity for carbon monoxide was determined with the single-breath technique following the European Respiratory Society/ATS guidelines,16 corrected by the hemoglobin value. The European Community for Steel and Coal values were referenced.17 Arterial blood gases were measured while breathing room air. The 6-minute walk distance (6MWD) was measured following the ATS guideline.18 Dyspnea was evaluated with the mMRC scale. FEV1, BMI, 6MWD, and mMRC values were integrated into the BODE index.19 Health status was assessed by the COPD assessment test (CAT).20 Comorbidities were classified with the Charlson index.21 Hospitalizations and all-cause mortality were recorded using information obtained from the family and electronic medical records as published previously.15 Cause of death was separated into two categories: all-cause and respiratory-related.
Statistical AnalysisWe complied with The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) standards of observational research22 and those of the Updated List of Essential Items for Reporting Diagnostic Accuracy Studies (STARD).23 Data are summarized as relative frequencies for categorical variables, mean (standard deviation) for normally distributed variables, and median (25–75th percentile) for non-normal data. As appropriate, comparisons were made using Pearson's Chi-squared test, the Kruskal–Wallis H test or the Mann–Whitney U test, and one-way Analysis of Variance or Student's t-test. Correlations are estimated using Spearman's or Pearson's linear coefficients. FEV1 and DLco were entered into the survival analysis as continuous and categorical variables. ppFEV1 thresholds according to the GOLD staging, DLco was categorized into 2 groups: DLco≥50 and <50% based on previous studies.13,15 Additionally, we confirmed the suitability of this cutoff point using ROC curve analysis and Youden's index. Standard Kaplan–Meier survival analyses were performed, and multivariable Cox models were built with additional clinical predictors, including age, gender, body mass index, %FEV1, DLco%, and comorbidities, selecting variables with a forward Wald method. Hazard ratios and 95% confidence intervals were calculated for each independent predictor. The Kaplan– Meier method estimated the mean survival (with the corresponding 95% confidence intervals) and the overall survival rates due to all respiratory causes. For all outcomes tested, and due to the relatively small number, the patients in GOLD spirometric stages 3 and 4 were combined into one single group. Statistical significance was set as a two-tailed p-value<0.05. Calculations were made with SPSS 29.0 (IBM SPSS, Armonk, NY).
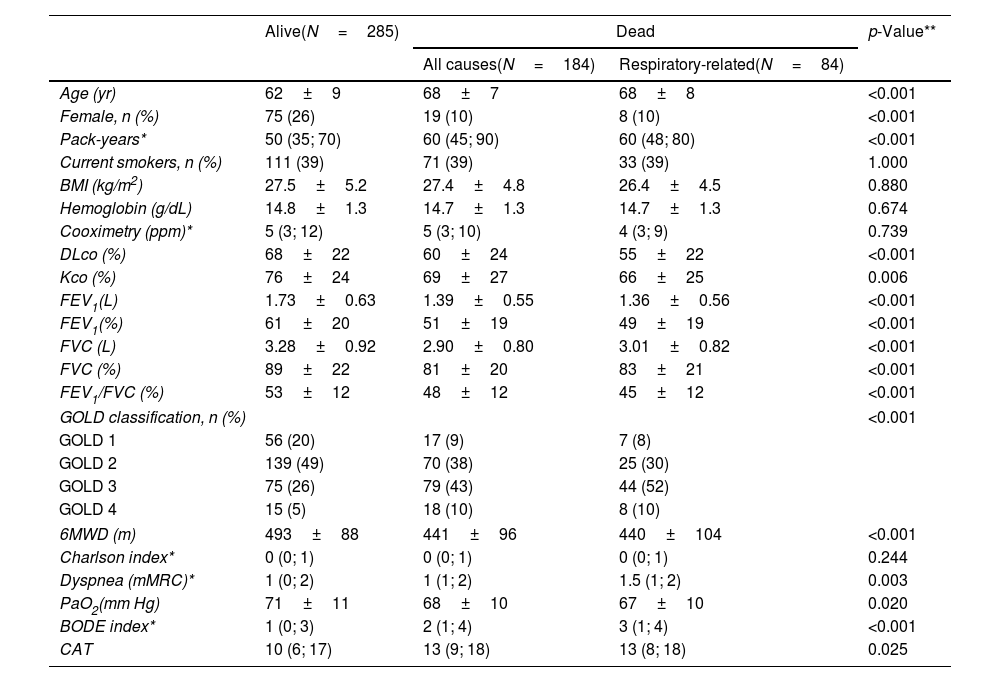
ResultsCharacteristics of CHAIN ParticipantsThe study population included 469 patients with COPD (20% female). There were 184 deaths (39.2%), with 84 due to respiratory causes (17.9%) during the follow-up period. The baseline characteristics of the patients according to vital status are shown in Table 1. The COPD patients who died were slightly older, primarily men, and had a greater pack-year smoking history but a similar proportion of current smokers than survivors. As expected, they had worse lung function, lower exercise capacity, higher dyspnea, and CAT and BODE index scores. However, the two groups had similar hemoglobin levels, BMI values, and comorbidities.
Baseline characteristics of the COPD patients included in the study, stratified by vital status.
| Alive(N=285) | Dead | p-Value** | ||
|---|---|---|---|---|
| All causes(N=184) | Respiratory-related(N=84) | |||
| Age (yr) | 62±9 | 68±7 | 68±8 | <0.001 |
| Female, n (%) | 75 (26) | 19 (10) | 8 (10) | <0.001 |
| Pack-years* | 50 (35; 70) | 60 (45; 90) | 60 (48; 80) | <0.001 |
| Current smokers, n (%) | 111 (39) | 71 (39) | 33 (39) | 1.000 |
| BMI (kg/m2) | 27.5±5.2 | 27.4±4.8 | 26.4±4.5 | 0.880 |
| Hemoglobin (g/dL) | 14.8±1.3 | 14.7±1.3 | 14.7±1.3 | 0.674 |
| Cooximetry (ppm)* | 5 (3; 12) | 5 (3; 10) | 4 (3; 9) | 0.739 |
| DLco (%) | 68±22 | 60±24 | 55±22 | <0.001 |
| Kco (%) | 76±24 | 69±27 | 66±25 | 0.006 |
| FEV1(L) | 1.73±0.63 | 1.39±0.55 | 1.36±0.56 | <0.001 |
| FEV1(%) | 61±20 | 51±19 | 49±19 | <0.001 |
| FVC (L) | 3.28±0.92 | 2.90±0.80 | 3.01±0.82 | <0.001 |
| FVC (%) | 89±22 | 81±20 | 83±21 | <0.001 |
| FEV1/FVC (%) | 53±12 | 48±12 | 45±12 | <0.001 |
| GOLD classification, n (%) | <0.001 | |||
| GOLD 1 | 56 (20) | 17 (9) | 7 (8) | |
| GOLD 2 | 139 (49) | 70 (38) | 25 (30) | |
| GOLD 3 | 75 (26) | 79 (43) | 44 (52) | |
| GOLD 4 | 15 (5) | 18 (10) | 8 (10) | |
| 6MWD (m) | 493±88 | 441±96 | 440±104 | <0.001 |
| Charlson index* | 0 (0; 1) | 0 (0; 1) | 0 (0; 1) | 0.244 |
| Dyspnea (mMRC)* | 1 (0; 2) | 1 (1; 2) | 1.5 (1; 2) | 0.003 |
| PaO2(mm Hg) | 71±11 | 68±10 | 67±10 | 0.020 |
| BODE index* | 1 (0; 3) | 2 (1; 4) | 3 (1; 4) | <0.001 |
| CAT | 10 (6; 17) | 13 (9; 18) | 13 (8; 18) | 0.025 |
Values are presented as mean and SD, n (%), or *median (IQR). **Comparisons between alive and dead COPD patients.
Abbreviations: BMI=body mass index; BODE=body mass index, airflow obstruction, dyspnea, and exercise capacity; CAT=COPD assessment test; DLco=diffusing capacity of the lungs for carbon monoxide; FEV1=forced expiratory volume in the first second; FVC=forced vital capacity; Kco=carbon monoxide transfer coefficient; 6MWD=six-minute walk distance.
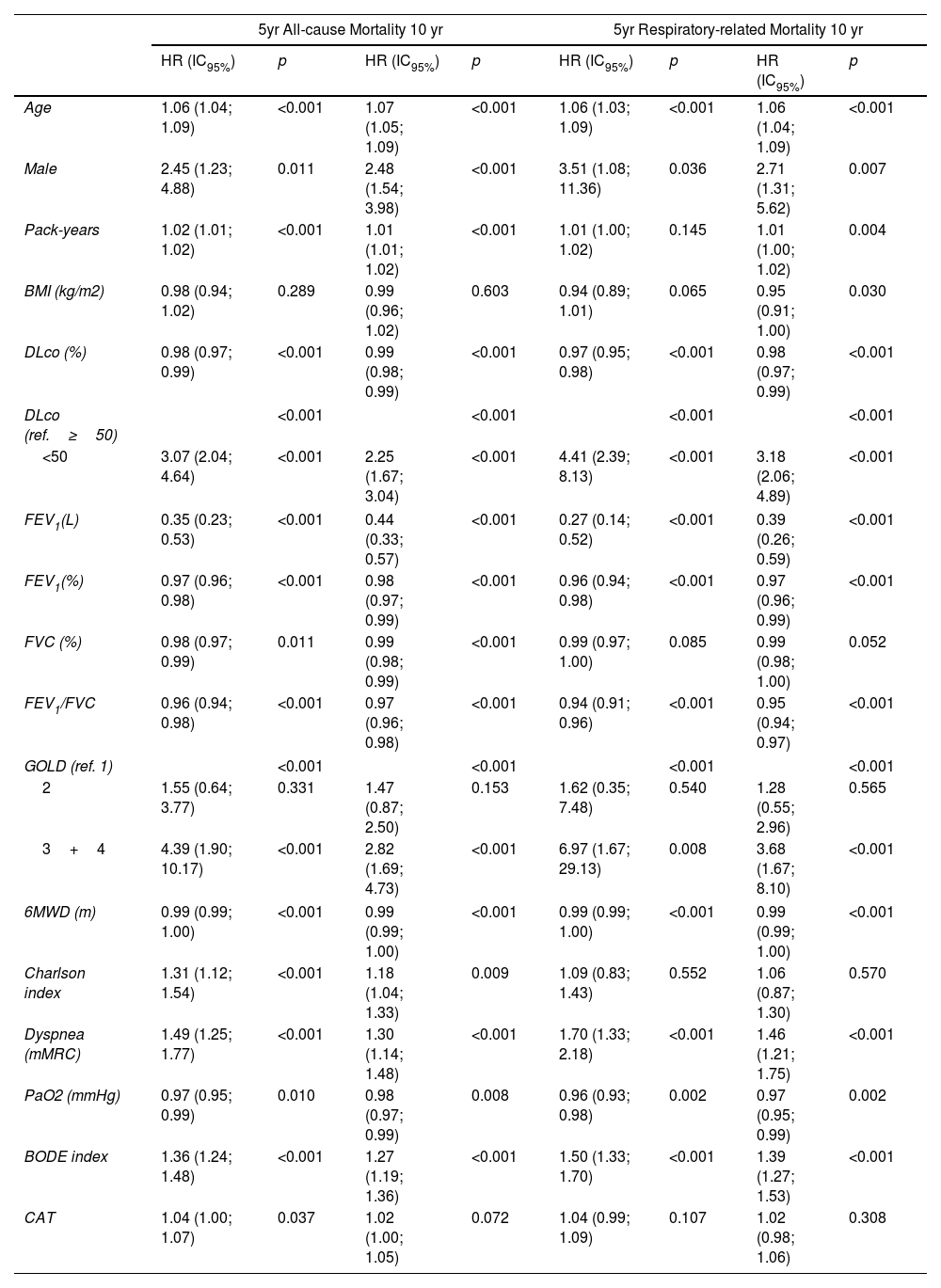
Table 2 shows the univariate predictors for all-cause mortality. Age, pack years, FEV1%, FVC%, DLco%, PaO2, 6MWD, Charlson index, dyspnea, and BODE index are related to mortality. Only DLco values lower than 50% are related to a significant risk of death.
Cox proportional univariate analysis of baseline variables associated with all-cause and respiratory-related mortality at 5 and 10 years.
| 5yr All-cause Mortality 10 yr | 5yr Respiratory-related Mortality 10 yr | |||||||
|---|---|---|---|---|---|---|---|---|
| HR (IC95%) | p | HR (IC95%) | p | HR (IC95%) | p | HR (IC95%) | p | |
| Age | 1.06 (1.04; 1.09) | <0.001 | 1.07 (1.05; 1.09) | <0.001 | 1.06 (1.03; 1.09) | <0.001 | 1.06 (1.04; 1.09) | <0.001 |
| Male | 2.45 (1.23; 4.88) | 0.011 | 2.48 (1.54; 3.98) | <0.001 | 3.51 (1.08; 11.36) | 0.036 | 2.71 (1.31; 5.62) | 0.007 |
| Pack-years | 1.02 (1.01; 1.02) | <0.001 | 1.01 (1.01; 1.02) | <0.001 | 1.01 (1.00; 1.02) | 0.145 | 1.01 (1.00; 1.02) | 0.004 |
| BMI (kg/m2) | 0.98 (0.94; 1.02) | 0.289 | 0.99 (0.96; 1.02) | 0.603 | 0.94 (0.89; 1.01) | 0.065 | 0.95 (0.91; 1.00) | 0.030 |
| DLco (%) | 0.98 (0.97; 0.99) | <0.001 | 0.99 (0.98; 0.99) | <0.001 | 0.97 (0.95; 0.98) | <0.001 | 0.98 (0.97; 0.99) | <0.001 |
| DLco (ref.≥50) | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| <50 | 3.07 (2.04; 4.64) | <0.001 | 2.25 (1.67; 3.04) | <0.001 | 4.41 (2.39; 8.13) | <0.001 | 3.18 (2.06; 4.89) | <0.001 |
| FEV1(L) | 0.35 (0.23; 0.53) | <0.001 | 0.44 (0.33; 0.57) | <0.001 | 0.27 (0.14; 0.52) | <0.001 | 0.39 (0.26; 0.59) | <0.001 |
| FEV1(%) | 0.97 (0.96; 0.98) | <0.001 | 0.98 (0.97; 0.99) | <0.001 | 0.96 (0.94; 0.98) | <0.001 | 0.97 (0.96; 0.99) | <0.001 |
| FVC (%) | 0.98 (0.97; 0.99) | 0.011 | 0.99 (0.98; 0.99) | <0.001 | 0.99 (0.97; 1.00) | 0.085 | 0.99 (0.98; 1.00) | 0.052 |
| FEV1/FVC | 0.96 (0.94; 0.98) | <0.001 | 0.97 (0.96; 0.98) | <0.001 | 0.94 (0.91; 0.96) | <0.001 | 0.95 (0.94; 0.97) | <0.001 |
| GOLD (ref. 1) | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| 2 | 1.55 (0.64; 3.77) | 0.331 | 1.47 (0.87; 2.50) | 0.153 | 1.62 (0.35; 7.48) | 0.540 | 1.28 (0.55; 2.96) | 0.565 |
| 3+4 | 4.39 (1.90; 10.17) | <0.001 | 2.82 (1.69; 4.73) | <0.001 | 6.97 (1.67; 29.13) | 0.008 | 3.68 (1.67; 8.10) | <0.001 |
| 6MWD (m) | 0.99 (0.99; 1.00) | <0.001 | 0.99 (0.99; 1.00) | <0.001 | 0.99 (0.99; 1.00) | <0.001 | 0.99 (0.99; 1.00) | <0.001 |
| Charlson index | 1.31 (1.12; 1.54) | <0.001 | 1.18 (1.04; 1.33) | 0.009 | 1.09 (0.83; 1.43) | 0.552 | 1.06 (0.87; 1.30) | 0.570 |
| Dyspnea (mMRC) | 1.49 (1.25; 1.77) | <0.001 | 1.30 (1.14; 1.48) | <0.001 | 1.70 (1.33; 2.18) | <0.001 | 1.46 (1.21; 1.75) | <0.001 |
| PaO2 (mmHg) | 0.97 (0.95; 0.99) | 0.010 | 0.98 (0.97; 0.99) | 0.008 | 0.96 (0.93; 0.98) | 0.002 | 0.97 (0.95; 0.99) | 0.002 |
| BODE index | 1.36 (1.24; 1.48) | <0.001 | 1.27 (1.19; 1.36) | <0.001 | 1.50 (1.33; 1.70) | <0.001 | 1.39 (1.27; 1.53) | <0.001 |
| CAT | 1.04 (1.00; 1.07) | 0.037 | 1.02 (1.00; 1.05) | 0.072 | 1.04 (0.99; 1.09) | 0.107 | 1.02 (0.98; 1.06) | 0.308 |
Definition of abbreviations: BMI=body mass index; BODE=body mass index, airflow obstruction, dyspnea, and exercise capacity; CAT=COPD assessment test; DLco=diffusing capacity of the lung for carbon monoxide; FEV1=forced expiratory volume in 1second; FVC=forced vital capacity; 6MWD=six-minute walk distance.
The univariate analysis for respiratory mortality showed that the BMI was significant only at 10 years, while the Charlson index failed to predict the event at 5 and 10 years. As expected, the respiratory function parameters, including DLco, increased their predictive capacity for this outcome (Table 2).
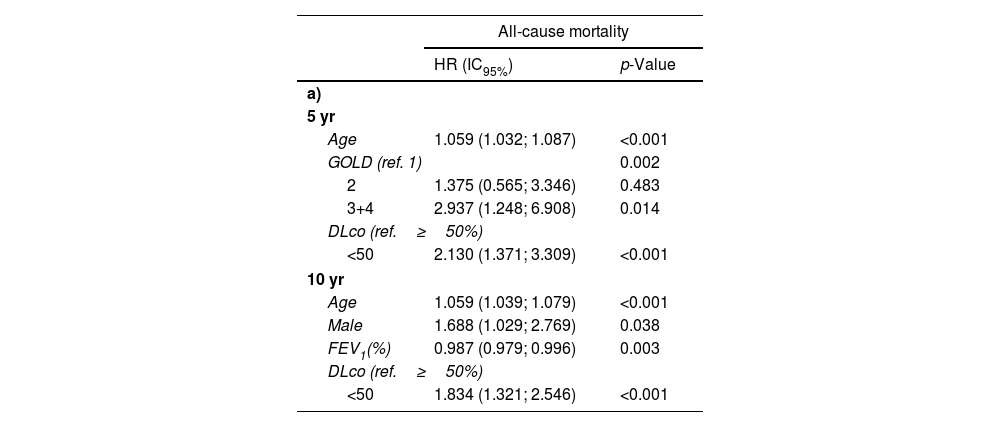
The multivariate Cox proportional analyses for all causes and respiratory mortality at five years (Table 3a and b) show that FEV1%, GOLD stages 3 and 4 (<50%), and DLco less than 50% were retained in the final statistical model after adjusting for age, sex, pack-years history, and BMI. The results are similar at ten years, except that the FEV1% was retained in the model, while the GOLD categorization was not (Table 3a and b).
Cox proportional multivariate survival analysis of baseline variables associated with all-cause (3a) and respiratory-related (3b) mortality.
| All-cause mortality | ||
|---|---|---|
| HR (IC95%) | p-Value | |
| a) | ||
| 5 yr | ||
| Age | 1.059 (1.032; 1.087) | <0.001 |
| GOLD (ref. 1) | 0.002 | |
| 2 | 1.375 (0.565; 3.346) | 0.483 |
| 3+4 | 2.937 (1.248; 6.908) | 0.014 |
| DLco (ref.≥50%) | ||
| <50 | 2.130 (1.371; 3.309) | <0.001 |
| 10 yr | ||
| Age | 1.059 (1.039; 1.079) | <0.001 |
| Male | 1.688 (1.029; 2.769) | 0.038 |
| FEV1(%) | 0.987 (0.979; 0.996) | 0.003 |
| DLco (ref.≥50%) | ||
| <50 | 1.834 (1.321; 2.546) | <0.001 |
| Respiratory-related mortality | ||
|---|---|---|
| HR (IC95%) | p-Value | |
| b) | ||
| 5 yr | ||
| Age | 1.067 (1.026; 1.110) | 0.001 |
| GOLD (ref. 1) | 0.008 | |
| 2 | 1.419 (0.306; 6.580) | 0.655 |
| 3+4 | 4.210 (0.988; 17.942) | 0.052 |
| DLco (ref.≥50%) | ||
| <50 | 2.739 (1.430; 5.244) | 0.002 |
| 10 yr | ||
| Age | 1.062 (1.034; 1.091) | <0.001 |
| GOLD (ref. 1) | 0.004 | |
| 2 | 1.113 (0.481; 2.578) | 0.803 |
| 3+4 | 2.399 (1.070; 5.379) | 0.034 |
| DLco (ref.≥50%) | ||
| <50 | 2.272 (1.433; 3.601) | <0.001 |
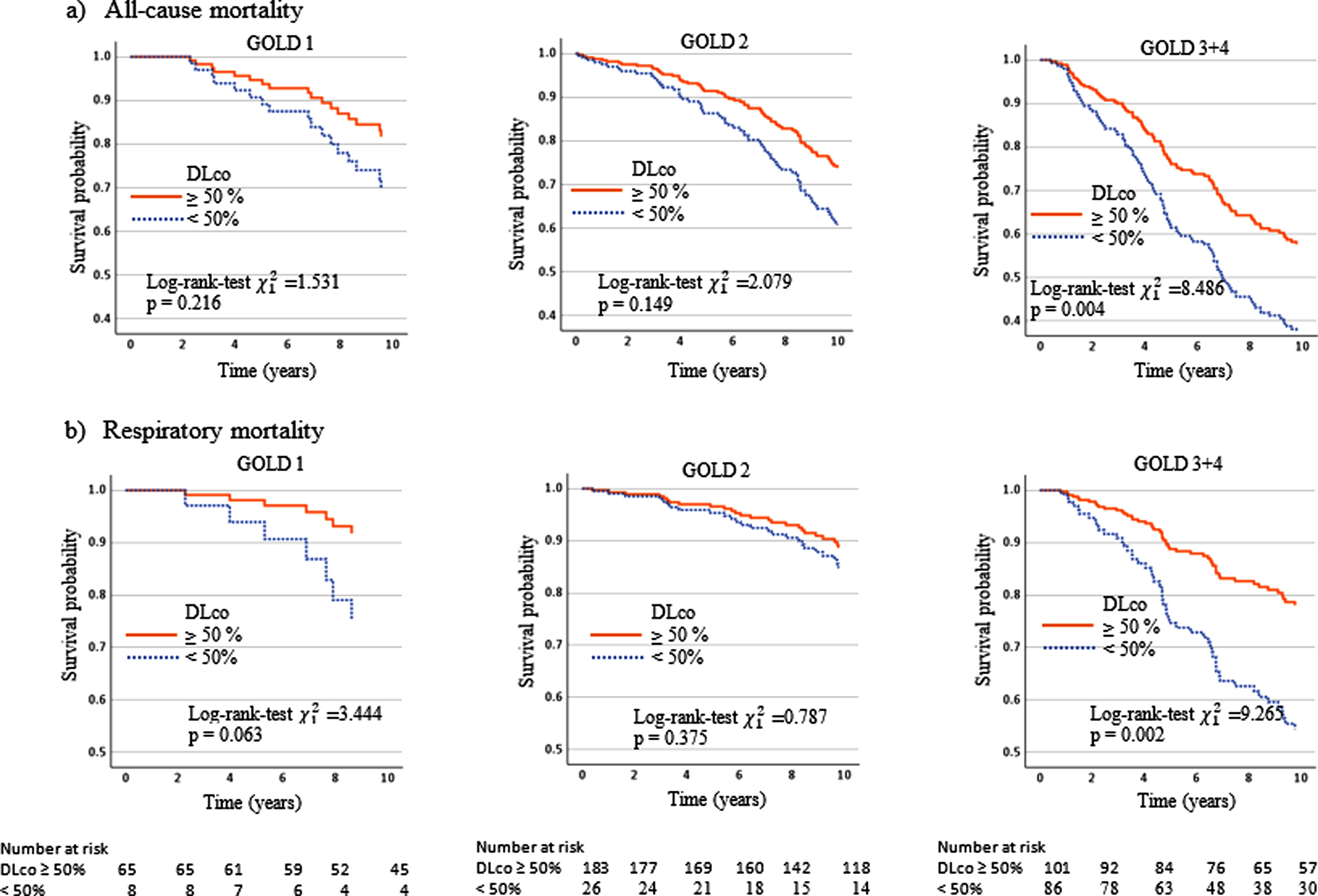
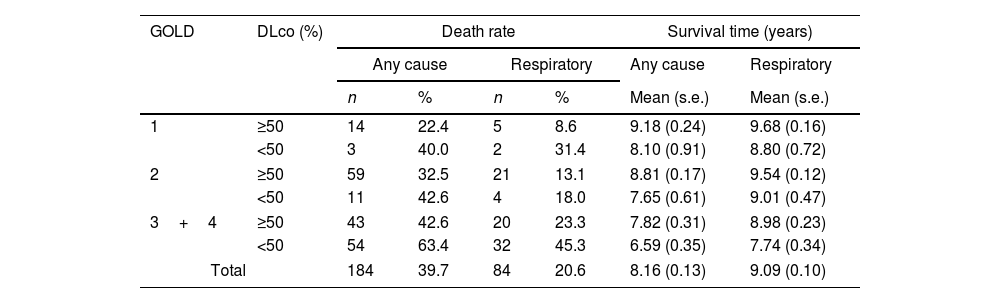
Table 4 and the Kaplan–Meier curves in Fig. 1a and b show that a DLco<50% improved the discriminative capacity of all GOLD stages for all-cause and respiratory mortality over the ten-year follow-up. For all-cause mortality, the survival time in patients with DLco≥50% was 8.6 (95% CI 8.3, 8.9) years, versus 6.9 (95% CI 6.4, 7.5) in patients with DLco<50% (p<0.001). For respiratory mortality, the survival time in patients with DLco>50% was 9.0 (95% CI 8.5, 9.4) years versus 7.7 (95% CI 7.1, 8.4) in patients with DLco<50% predicted (p<0.002).
Ten years mortality combining GOLD stages and DLco value <50% predicted.
| GOLD | DLco (%) | Death rate | Survival time (years) | ||||
|---|---|---|---|---|---|---|---|
| Any cause | Respiratory | Any cause | Respiratory | ||||
| n | % | n | % | Mean (s.e.) | Mean (s.e.) | ||
| 1 | ≥50 | 14 | 22.4 | 5 | 8.6 | 9.18 (0.24) | 9.68 (0.16) |
| <50 | 3 | 40.0 | 2 | 31.4 | 8.10 (0.91) | 8.80 (0.72) | |
| 2 | ≥50 | 59 | 32.5 | 21 | 13.1 | 8.81 (0.17) | 9.54 (0.12) |
| <50 | 11 | 42.6 | 4 | 18.0 | 7.65 (0.61) | 9.01 (0.47) | |
| 3+4 | ≥50 | 43 | 42.6 | 20 | 23.3 | 7.82 (0.31) | 8.98 (0.23) |
| <50 | 54 | 63.4 | 32 | 45.3 | 6.59 (0.35) | 7.74 (0.34) | |
| Total | 184 | 39.7 | 84 | 20.6 | 8.16 (0.13) | 9.09 (0.10) | |
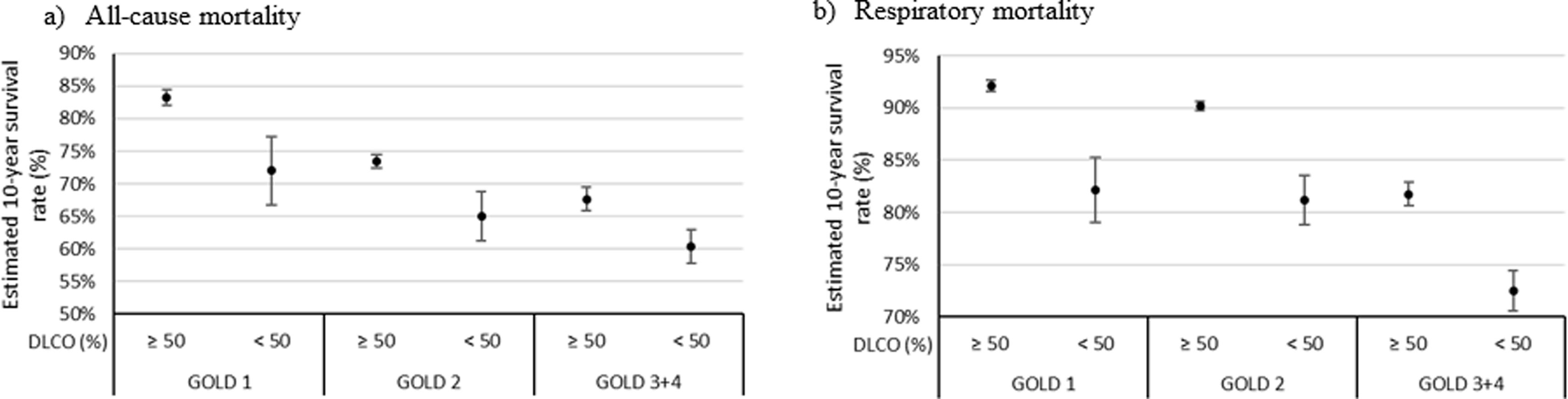
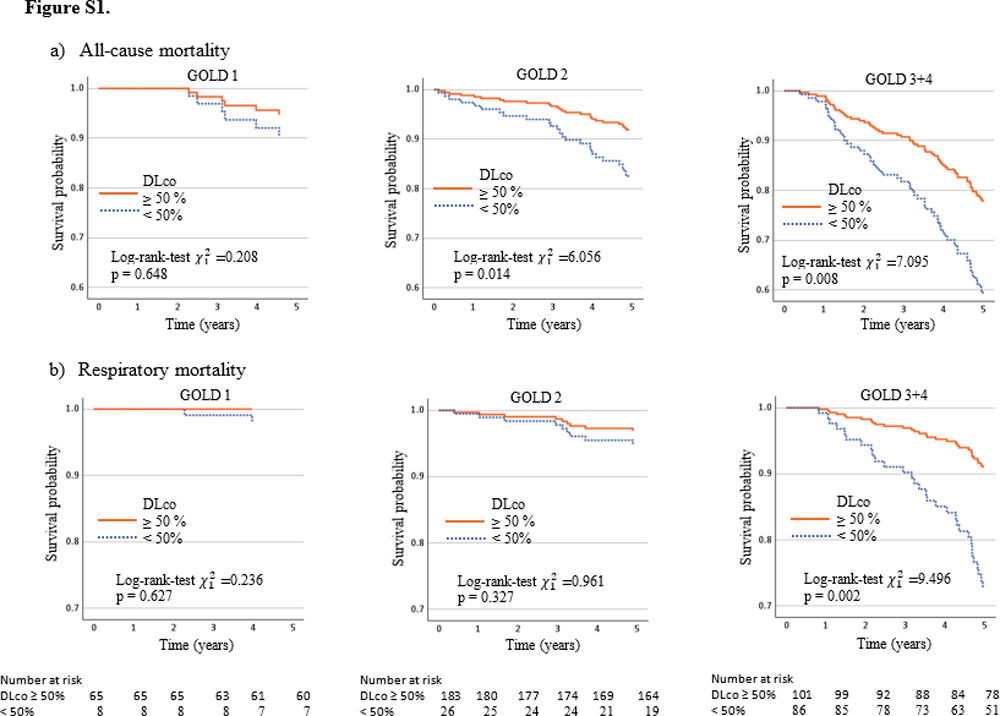
The survival rate for all-cause mortality was 57.4% in GOLD 3–4 patients with DLco≥50% versus 36.6% in patients with DLco<50% (p=0.004). The survival rate for respiratory cause was 76.7% in GOLD 3–4 patients with DLco≥50% versus 54.7 in patients with DLco<50% (p=0.002). The survival rate for all-cause and respiratory causes in patients GOLD 1 was 17.6% and 22.8% lower in patients with DLco<50% compared with patients with DLco>50% (p=0.216 and p=0.063, respectively). GOLD 2's differences were also lower: 10.1% and 4.9% for overall and respiratory mortality (p=0.163 and p=0.375, respectively). Models associated with all-cause and respiratory mortality and survival rate estimated at ten years are shown in Fig. 2. The Kaplan–Meier curves and models of survival rate showed a similar trend in all GOLD stages for all-cause and respiratory mortality over five years of follow-up (Figs. S1 and S2).
Estimated 10-year all-cause (a) and respiratory (b) mortality rates from the models shown in Table 3.
DLco<50% was also associated with a significant increase in the BODE index score at all GOLD stages: one BODE point in 1–2 and two BODE points in 3/4 (Fig. S3).
External Reproducibility in the Canadian CohortThe reproducibility of our observations was tested in the Kingston COPD cohort (Canada) with a similar annual follow-up protocol.13 Three hundred patients with COPD fulfilled the same inclusion criteria used in our study. After ten years of follow-up, there were 175 deaths (38.3%), 60 of which were due to respiratory causes. Table S1 shows the baseline characteristics of the patients according to their vital status.
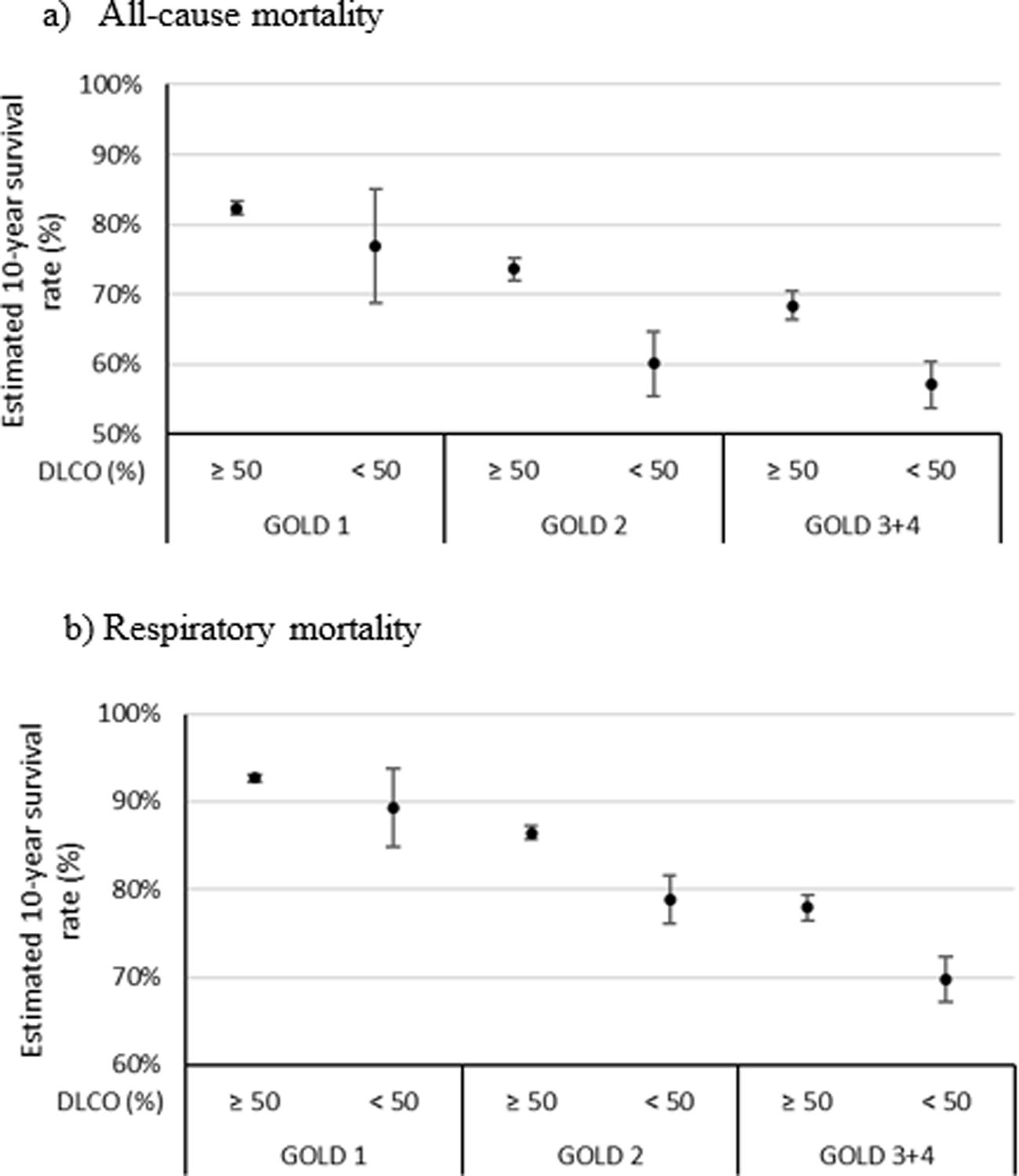
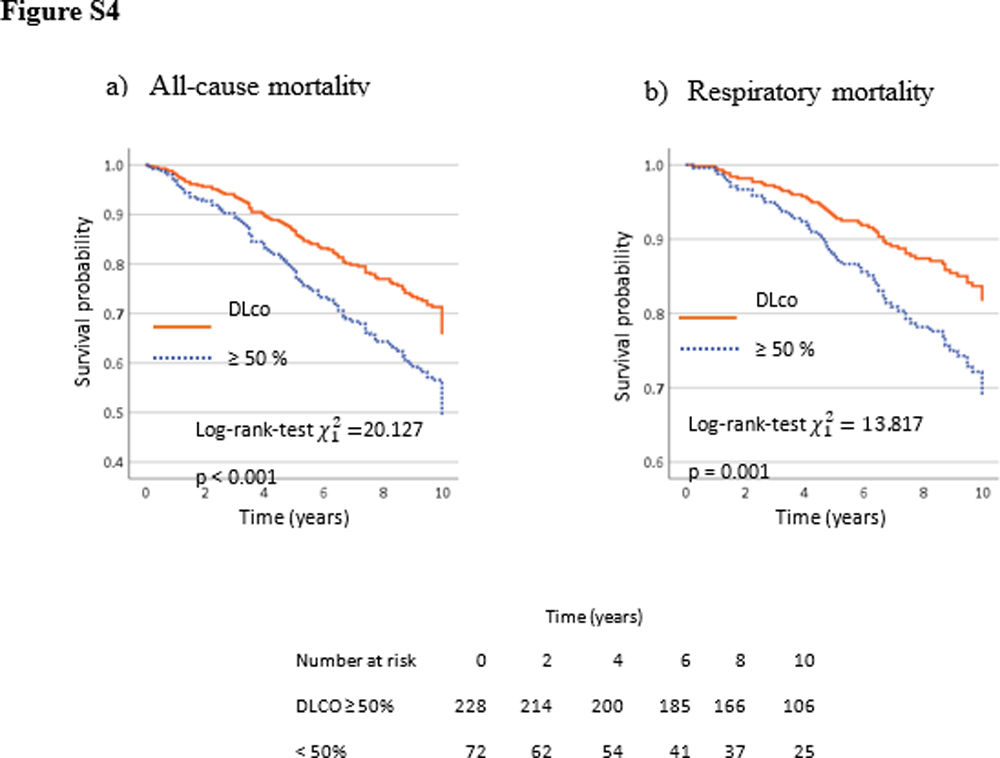
The multivariate Cox proportional analyses for all causes and respiratory mortality at ten years show that FEV1%, GOLD stages 3 and 4 (<50%), and DLco<50% were retained in the final statistical model after adjusting for age, sex, pack-years, and BMI. DLco<50% was an independent predictor of all-cause and respiratory mortality; DLco, HR=1.68 (95%CI 1.10–2.58, p<0.016) and 1.831 (95%CI 1.06–3.18, p<0.032), respectively (Table S2). The Kaplan–Meier curves and models of survival rate adding DLCO<50% into all GOLD obstruction stages showed a similar profile as that observed in the CHAIN cohort (Fig. S4 and Fig. 3). In addition to reproducing the models on the Kingston cohort (Table S3), an external validation of the obtained models has been performed using the CHAIN cohort (Table 3), applying them to the data from the Kingston cohort. The comparison of the estimated cumulative risk and the estimated probability of survival at 2, 5, and 10 years between individuals who died and those who did not during the study period, both for overall mortality and respiratory mortality, shows significant differences (p<0.001).
DiscussionThis 10-year prospective, multicenter, observational study shows that DLco<50% predicted is a strong and independent predictor of all-cause and respiratory mortality in patients with COPD. Adding DLco to the GOLD FEV1% staging provides a more robust categorization of the risk of a negative vital outcome, predicting the risk of death better than FEV1. These results were externally validated in the Kingston Canadian COPD cohort. Our results support the growing recognition that DLco is an important physiological biomarker that should be more frequently requested and clinically valued by those involved in the care of COPD.
DLco and MortalityOur results expand previous work demonstrating that DLco predicts all-cause mortality. Boutou et al.12 and Balasubramanian and colleagues14 followed patients over a mean follow-up of 5 and 6.5 years and reported that the DLco% performed slightly better than FEV1%. Despite differences in the severity of airway obstruction between the studies, the DLco<50% threshold demonstrated a strong discriminative capacity, a finding herein confirmed (Table 3). The relationship between DLco and survival in patients with COPD is not linear, with threshold values at which the risk of death increases sharply.12 Importantly, neither Boutou et al.12 nor Balasubramanian et al.14 investigated a putative association between low DLco and specific respiratory mortality. In the current study, we show, for the first time, that this is the case (Tables 2 and 3), suggesting a mechanistic relationship between physiological impairment measured by the DLco and the organ system specific cause of death.
Adding DLco to the GOLD Spirometric ClassificationRecent studies propose alternatives to the FEV1% for assessing COPD severity, as FEV1% does not correct well for lung size, race/ethnicity, or concomitant restrictive physiology. Using the FEV1z-score or the FEV1/FVC, adjustments have24–28 marginally, if at all, improved mortality prediction. Although previous studies suggest that DLco should be considered in assessing and classifying patients with COPD,12,14 none evaluated if it adds predictive power to the current GOLD spirometric classification. Our findings do support an additive prognostic role of DLco when integrated GOLD spirometric stages; of note, a greater impact was observed in GOLD stages 3–4 in whom patient's survival decreased by more than a year over ten years (Fig. 1). This finding was confirmed in the Canadian cohort with a similarly long observation period (Fig. 3).
In patients with GOLD 1 and 2 with DLco<50%, the mortality risk increased compared to simple spirometric staging, but the difference was not statistically significant. The lack of statistical power in these milder stages is likely due to the small sample size of patients in these stages in the CHAIN cohort. This explanation is supported by results from a previous study from our group, including a total of 360 patients from the BODE, Kingston, and CHAIN cohorts in the GOLD I stage, which showed that a DLco value<60% predicted was independently associated with an increased risk of death.13 The contention that adding DLco to the milder stages of GOLD is clinically useful is supported by the finding that the overall survival in patients with GOLD stages 2 and DLco<50% was the same as that of patients classified with GOLD spirometric stage 3/4 (57.4%) (Table 4).
The validity of our results is indirectly supported by the observation that DLco levels<50% were associated with a significant increase in the BODE index from 1 point in milder GOLD stages (1/2) to 2 points in stages 3/4. Changes in the BODE index of more than 1 point have been associated with increases in respiratory and all-cause mortality.19
A key property of any clinically useful classification of COPD severity should include its ability to predict the risk of all-cause and respiratory mortality.2,28 Measurement of the DLco has become widely available due to reliable portable systems that have low intra-subject variability, expanding their use globally.14,29 The strong signal linking a low DLCO to mortality and FEV1 likely stems from several sources. A low DLCO may reflect the severity of comorbidities known to be negatively associated with survival and be present in patients with COPD, such as interstitial lung disease30 and pulmonary hypertension.31,32 Low DLCO is associated with resting and exercise-related hypoxemia, and lung hyperinflation (REF), all physiological disturbances associated with poorer prognosis.5
Study LimitationsThis study has some limitations. First, other lung function parameters shown to be significant predictors of mortality, such as the inspiratory to total lung capacity ratio (IC/TLC), were not analyzed. However, lung volume measurement is less accessible in routine clinical practice and has a stronger collinearity with the FEV1 than DLco.33 Second, we used the European Community for Steel and Coal reference values, not the GLI's.34 When the study was planned, the investigators agreed to use the former; further, our group demonstrated a strong correlation for DLco between both predictive reference values.35 Third, the percentage of women in the external validation cohort is higher compared to the main study cohort. However, this reinforces the prognostic role of DLco regardless of sex. Fourth, the results were obtained from patients recruited in specialty clinics in the hospital setting and may not be generalizable to COPD patients assessed in primary care. Nevertheless, it is likely that the findings are comparable, since patients at all stages of GOLD spirometric impairment were included in the study. Finally, one could speculate that the patient sample is relatively small to complete solid subgroup analyses (stages). However, the follow-up time of the cohort has been long, with a sufficient number of deaths, which made it possible to demonstrate a significant predictive capacity of DLco. In fact, in our external validation cohort, we observed similar results, albeit with a smaller sample size but a prolonged follow-up period. In summary, the combined results of 10-year multicenter studies of patients with COPD from two continents showed that a DLco<50% significantly increases the power of the GOLD FEV1% staging in predicting all-cause and respiratory-related mortality. Since moderate-to-severe decrements in DLCO also predict COPD-related morbidity10 and impaired quality-of-life,8 DLCO should be routinely requested and incorporated into risk prediction in this patient population.
Authors’ ContributionsThe above-listed authors attest that they contributed substantially to the study's conception and design, acquisition, analysis, and interpretation of the data, drafting the article or revising it critically for important intellectual content, and final approval of the version to be submitted for revision. All authors had full access to all the data in the study and accepted responsibility for submitting this work.
Generative AIWe have not used artificial intelligence in the writing of this article.
Funding/SupportThe study was partly funded by an unrestricted grant from Astra-Zeneca and Menarini (both companies had no role in the study's design, data collection and analysis, or manuscript preparation). Funds were also provided by the COPD research program of the Spanish Respiratory Society (PII de EPOC of SEPAR).
Conflict of InterestsCarlos Amado and José Luis López-Campos are members of the Editorial board of Archivos de Bronconeumología and declare that they have remained outside the evaluation and decision-making process in relation to this article. The rest of the authors declare no conflict of interest.