The Global Initiative for Obstructive Lung Disease (GOLD) recommends lung cancer screening for patients with Chronic Obstructive Pulmonary Disease (COPD), but data is lacking regarding results of screening in this high-risk population. The main goal of the present work is to explore if lung cancer screening with Low Dose Chest Tomography (LDCT) in people with COPD, allows lung cancer (LC) diagnosis in early stages with survival compatible with curative state.
MethodsThis is a post hoc exploratory analysis. Pamplona International Early Lung Cancer Action Program (P-IELCAP) participants with a GOLD defined obstructive pattern (post bronchodilator FEV1/FVC<0.70) were selected for analysis. The characteristics of those who developed LC and their survival are described. A Cox proportional analysis explored the factors associated with LC diagnosis.
ResultsEight hundred and sixty-five patients (77% male, 93% in spirometric GOLD stage 1+2) were followed for 102±63 months. LC prevalence was 2.6% at baseline, with an annual LC diagnosis rate of 0.68%. Early-stage tumors predominated (74%) with a median survival (25–75th percentiles) of 139 (76–185) months. Cumulative tobacco exposure, FEV1%, and emphysema were the main predictors of an LC diagnosis. Eight (11%) patients with COPD had a second LC, most of them in early stage (92%), and 6 (8%) had recurrence. Median survival (25–75th percentiles) in these patients was 168 (108–191) months.
ConclusionsLung cancer screening of selected high-risk participants with COPD allowed the LC diagnosis in early stages with survival compatible with curative state.
The Global Initiative for Obstructive Lung Disease (GOLD) recommends annual lung cancer screening with low dose chest tomography (LDCT) for COPD patients 50–80 years of age with a 20-pack year smoking history who currently smoke, or who have quit smoking within the past 15 years.1 Patients with COPD, especially the emphysema phenotype and those with worse lung function, have a higher risk of developing lung cancer2–5 and tend to develop more aggressive cancers.6 Most of the information available in the literature on the potential impact of screening COPD patients for LC with LDCT, comes from retrospective studies,7 including an ad hoc analysis of the National Lung Screening Trial (NLST) database.8 Data from these studies suggests that appropriately selected COPD patients benefit from screening in several ways, including tumor stage shift toward an early stage at diagnosis and mortality.6 Those with spirometric GOLD stages 1–2, emphysema, age>60 years old, low body mass index (BMI) (<25), a significant smoking history (>60 pack-years history) and a family history of LC may benefit the most.9 Unfortunately, appropriately designed studies exploring the impact of screening in COPD have not been published. Therefore, the main goal of the present work is to explore if LC screening with LDCT in people with COPD, allow LC diagnosis in early stages with survival compatible with curative state.
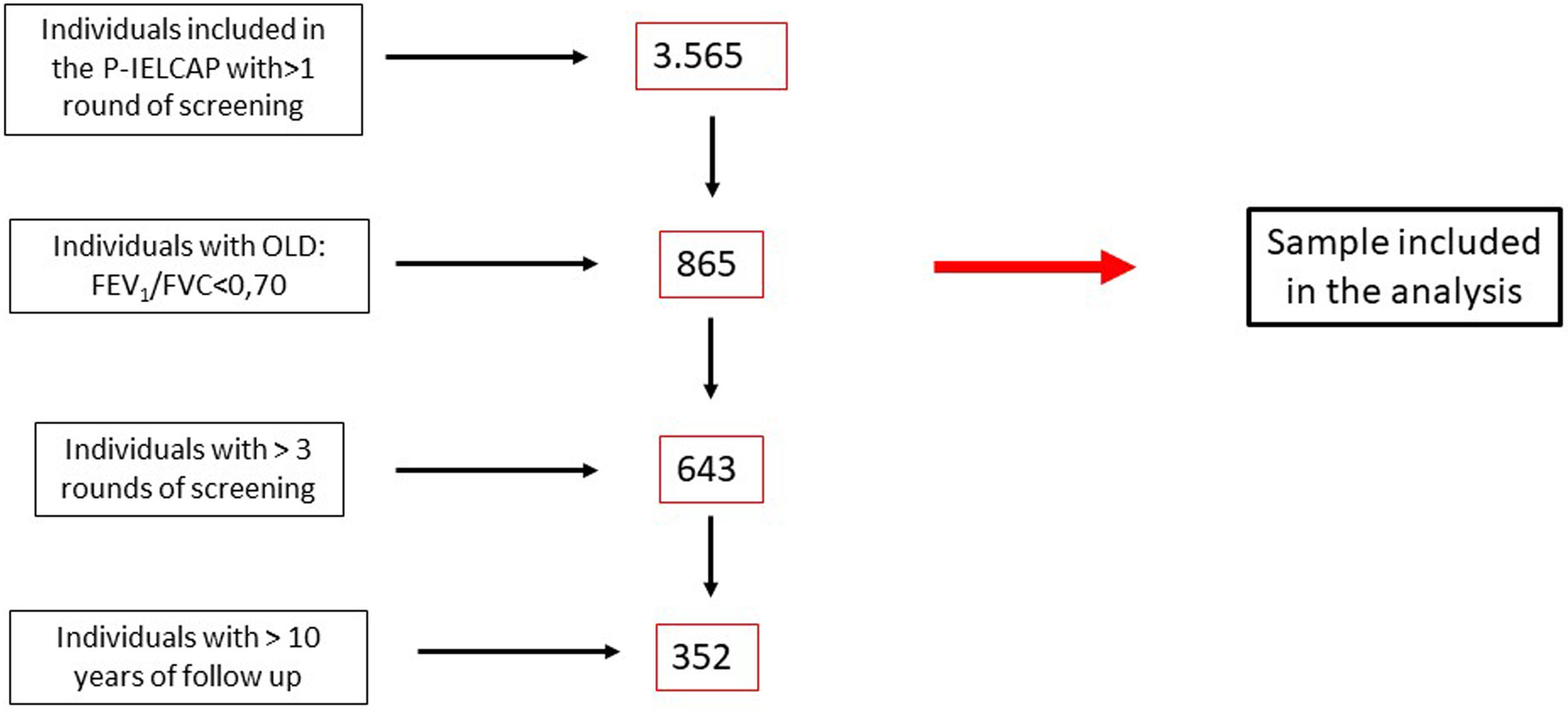
MethodsThis is a post hoc exploratory analysis. We conducted an observational study of outcomes in people with COPD prospectively recruited within the P-IELCAP between 2000 and 2019 and followed annually for a mean of 8.5 years. LC diagnosis, tumor histology and stage, LC prevalence (first round of screening), LC annual incidence rate (subsequent rounds of screening), overall survival, recurrence rate, metachronous primaries, and cause of death were recorded. As previously described10 this lung cancer screening program (LCSP) included men and women, 40 years of age or older, who were current or former smokers with a smoking history of at least 10 pack-years, with no lung cancer symptoms at the time of enrollment. All participants were equally invited to participate in the program if they fulfilled the inclusion criteria. All participants were systematically asked if they continued smoking and if they did, smoking cessation was offered. As described in Fig. 1 we only included in this analysis those active or former smokers with airway obstruction according to the GOLD recommendations (post bronchodilator forced expiratory volume in 1s/forced vital capacity (FEV1/FVC) ratio<70%). LDCT and spirometry were performed at baseline and annually during follow-up, according to the established I-ELCAP protocol.10 Initially, participants with no nodules or with non-calcified nodules<5mm on LDCT were invited to an annual repeat screening. Positive results (non-calcified nodules initially≥5mm and later≥6mm) required further study depending on nodule size and characteristics, including PET-CT, antibiotic treatment, short term LDCT follow up at one or three months, and/or percutaneous needle or surgical biopsy. Individuals whose tests did not suggest or confirm malignancy were invited to continue annual screening. Participants diagnosed with LC were closely followed according to the institutional protocol and included in a LC registry. There was no limit on the number of screening rounds. Emphysema was determined visually by two independent expert radiologists (GB and AE). The University of Navarra's ethics committee approved the study protocol and subsequent revisions (CUN CEIC 04/05/2000; current ref: 028/2012 mod3, 02/06/2022), and all subjects signed an informed consent prior to enrollment. Each patient status (live, dead, LC diagnosis) was followed up yearly according to the LCS protocol, until the patient decides to abandon the program. All patient with LC diagnosis were included in a LC registry and information regarding their vital status, recurrence, metachronous LC were recorded in all of them. Medical records were reviewed to obtain mortality data on all patients.
Statistical analysisTo explore the normality of the data distribution of the evaluated parameters, we used the Kolmogorov–Smirnov test. Relative frequencies for categorical variables and mean (standard deviation [SD]) for normally distributed variables (only normally distributed variables were found) were recorded. Comparison of relative frequencies was assessed by the Chi-square test and comparison of means using the Student t test. The Cox proportional hazards method was used to assess the risk of being diagnosed with LC using age, sex, pack-years of smoking, BMI, FEV1%, DLCO and emphysema as predictors of an LC diagnosis. Kaplan–Meier survival analysis explored the independent association of those with and without an LC diagnosis, with all-cause mortality. A log rank test explored differences between groups. A two-tailed p-value≤0.05 was considered statistically significant. All data were analyzed using SPSS software v.26.0, Chicago, U.S.A.
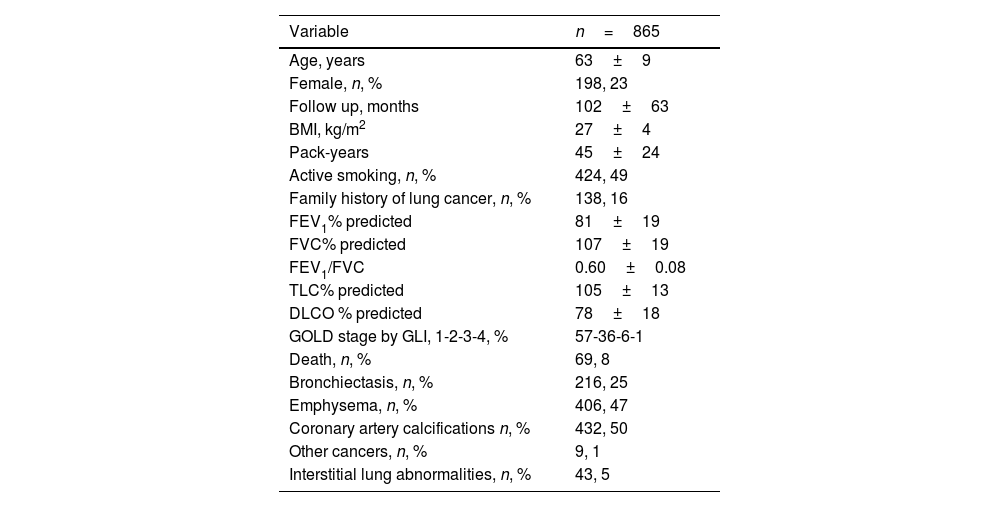
ResultsTable 1 shows general characteristics of the COPD cohort participating in P-IELCAP and included in the present study. Participants were mostly male with mild to moderate degrees of airflow obstruction (AO). They were slightly overweight, with a significant smoking history, and approximately half of them continued to smoke. Sixteen percent of the participants with COPD had a family history of LC. Thirty four percent of those that continued to smoke (145) accepted to participate at least once or more times in a Smoking Cessation Program. Mean follow up was 8.5 years and only 8% died during follow up.
Characteristics of participants included in the analysis.
| Variable | n=865 |
|---|---|
| Age, years | 63±9 |
| Female, n, % | 198, 23 |
| Follow up, months | 102±63 |
| BMI, kg/m2 | 27±4 |
| Pack-years | 45±24 |
| Active smoking, n, % | 424, 49 |
| Family history of lung cancer, n, % | 138, 16 |
| FEV1% predicted | 81±19 |
| FVC% predicted | 107±19 |
| FEV1/FVC | 0.60±0.08 |
| TLC% predicted | 105±13 |
| DLCO % predicted | 78±18 |
| GOLD stage by GLI, 1-2-3-4, % | 57-36-6-1 |
| Death, n, % | 69, 8 |
| Bronchiectasis, n, % | 216, 25 |
| Emphysema, n, % | 406, 47 |
| Coronary artery calcifications n, % | 432, 50 |
| Other cancers, n, % | 9, 1 |
| Interstitial lung abnormalities, n, % | 43, 5 |
Abbreviations: BMI: body mass index, FEV1: forced expiratory volume in first second, FVC: forced vital capacity, TLC: total lung capacity, DLCO: diffusion capacity for carbon monoxide, GOLD: Global Initiative for Obstructive Lung Disease, GOLD stages by FEV1: 1 – >80%, 2 – 50–79%, 3 – 30–49%, 4 – <30%, GLI: global lung initiative.
Quantitative variables expressed as mean and standard deviations (SD). Qualitative variables expressed as number (n) and percentage (%).
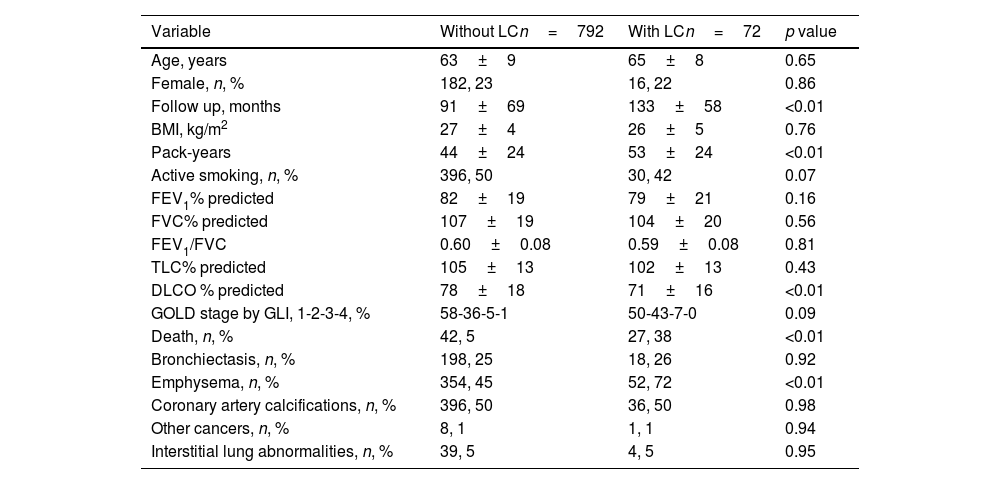
Lung cancer was diagnosed in 72 participants, 8% of the cohort. The characteristics of those with and without LC are described in Table 2. Patients with LC had longer follow up, smoked more, had lower DLCO values, and more emphysema. Not surprisingly, patients with LC had a higher mortality.
Characteristics of participants with and without LC.
| Variable | Without LCn=792 | With LCn=72 | p value |
|---|---|---|---|
| Age, years | 63±9 | 65±8 | 0.65 |
| Female, n, % | 182, 23 | 16, 22 | 0.86 |
| Follow up, months | 91±69 | 133±58 | <0.01 |
| BMI, kg/m2 | 27±4 | 26±5 | 0.76 |
| Pack-years | 44±24 | 53±24 | <0.01 |
| Active smoking, n, % | 396, 50 | 30, 42 | 0.07 |
| FEV1% predicted | 82±19 | 79±21 | 0.16 |
| FVC% predicted | 107±19 | 104±20 | 0.56 |
| FEV1/FVC | 0.60±0.08 | 0.59±0.08 | 0.81 |
| TLC% predicted | 105±13 | 102±13 | 0.43 |
| DLCO % predicted | 78±18 | 71±16 | <0.01 |
| GOLD stage by GLI, 1-2-3-4, % | 58-36-5-1 | 50-43-7-0 | 0.09 |
| Death, n, % | 42, 5 | 27, 38 | <0.01 |
| Bronchiectasis, n, % | 198, 25 | 18, 26 | 0.92 |
| Emphysema, n, % | 354, 45 | 52, 72 | <0.01 |
| Coronary artery calcifications, n, % | 396, 50 | 36, 50 | 0.98 |
| Other cancers, n, % | 8, 1 | 1, 1 | 0.94 |
| Interstitial lung abnormalities, n, % | 39, 5 | 4, 5 | 0.95 |
Abbreviations: BMI: body mass index, FEV1: forced expiratory volume in first second, FVC; forced vital capacity, TLC: total lung capacity, DLCO: diffusion capacity for carbon monoxide, GOLD: Global Initiative for Obstructive Lung Disease, GOLD stages by FEV1: 1 – >80%, 2 – 50–79%, 3 – 30–49%, 4 – <30%, GLI: global lung initiative.
Quantitative variables expressed as mean and standard deviations (SD).
Qualitative variables expressed as number (n) and percentage (%).
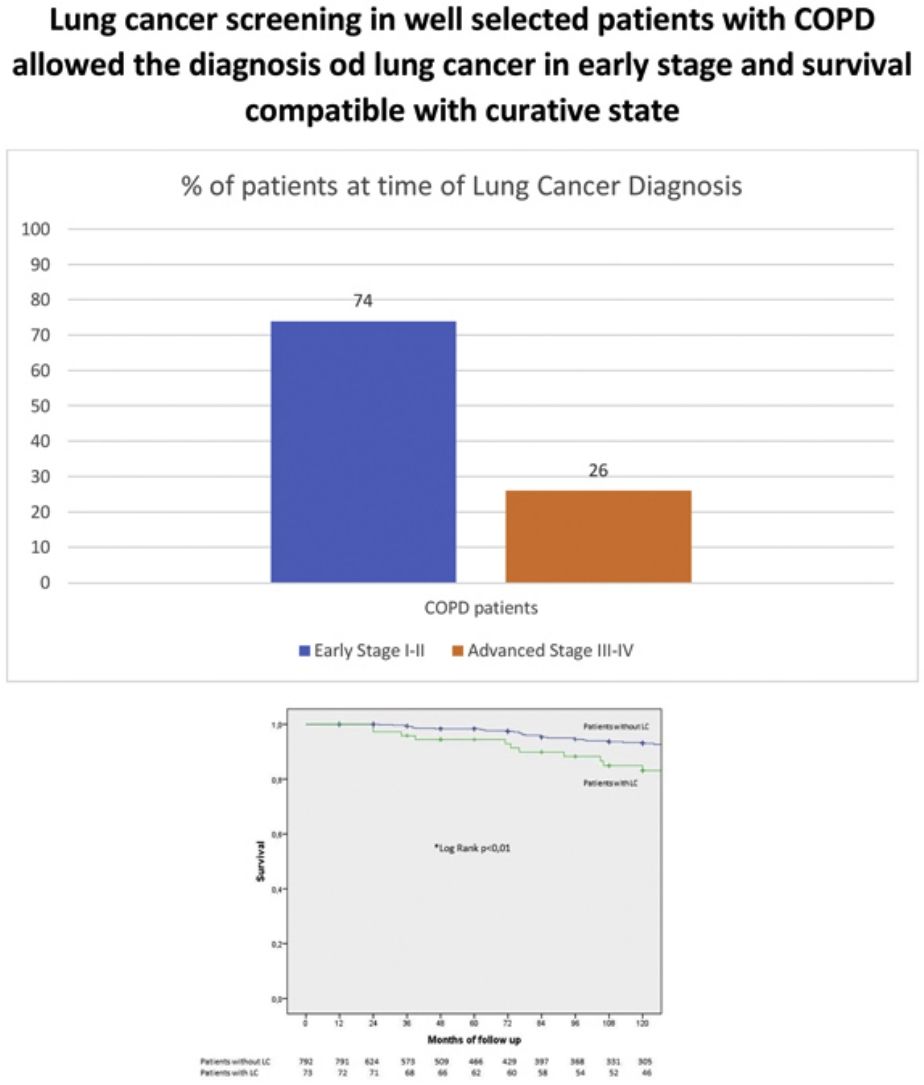
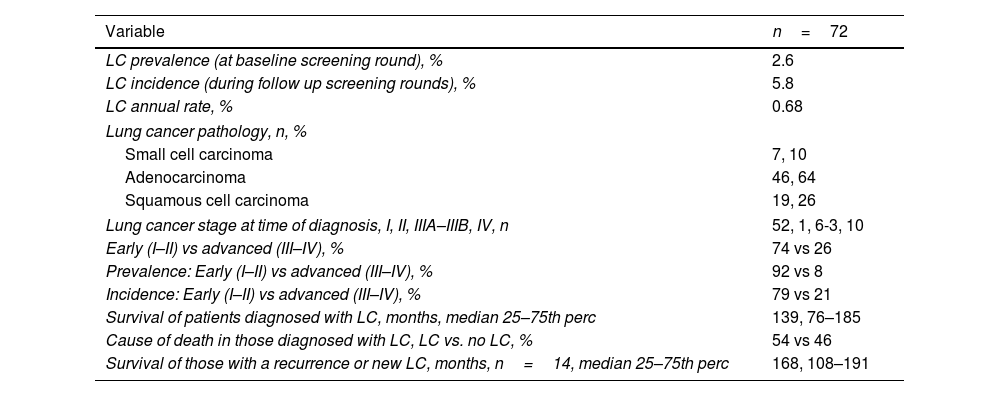
Tumor specific variables are described in Table 3. The prevalence of LC at baseline was 2.6%. The incidence of LC in subsequent screening rounds was 5.8% with an annual detection rate of 0.68%. The most frequent histology was adenocarcinoma followed by squamous cell carcinoma. Most patients with LC had early-stage disease and prolonged survival. Only half of those diagnosed with LC died from cancer.
Characteristics of participants diagnosed with LC.
| Variable | n=72 |
|---|---|
| LC prevalence (at baseline screening round), % | 2.6 |
| LC incidence (during follow up screening rounds), % | 5.8 |
| LC annual rate, % | 0.68 |
| Lung cancer pathology, n, % | |
| Small cell carcinoma | 7, 10 |
| Adenocarcinoma | 46, 64 |
| Squamous cell carcinoma | 19, 26 |
| Lung cancer stage at time of diagnosis, I, II, IIIA–IIIB, IV, n | 52, 1, 6-3, 10 |
| Early (I–II) vs advanced (III–IV), % | 74 vs 26 |
| Prevalence: Early (I–II) vs advanced (III–IV), % | 92 vs 8 |
| Incidence: Early (I–II) vs advanced (III–IV), % | 79 vs 21 |
| Survival of patients diagnosed with LC, months, median 25–75th perc | 139, 76–185 |
| Cause of death in those diagnosed with LC, LC vs. no LC, % | 54 vs 46 |
| Survival of those with a recurrence or new LC, months, n=14, median 25–75th perc | 168, 108–191 |
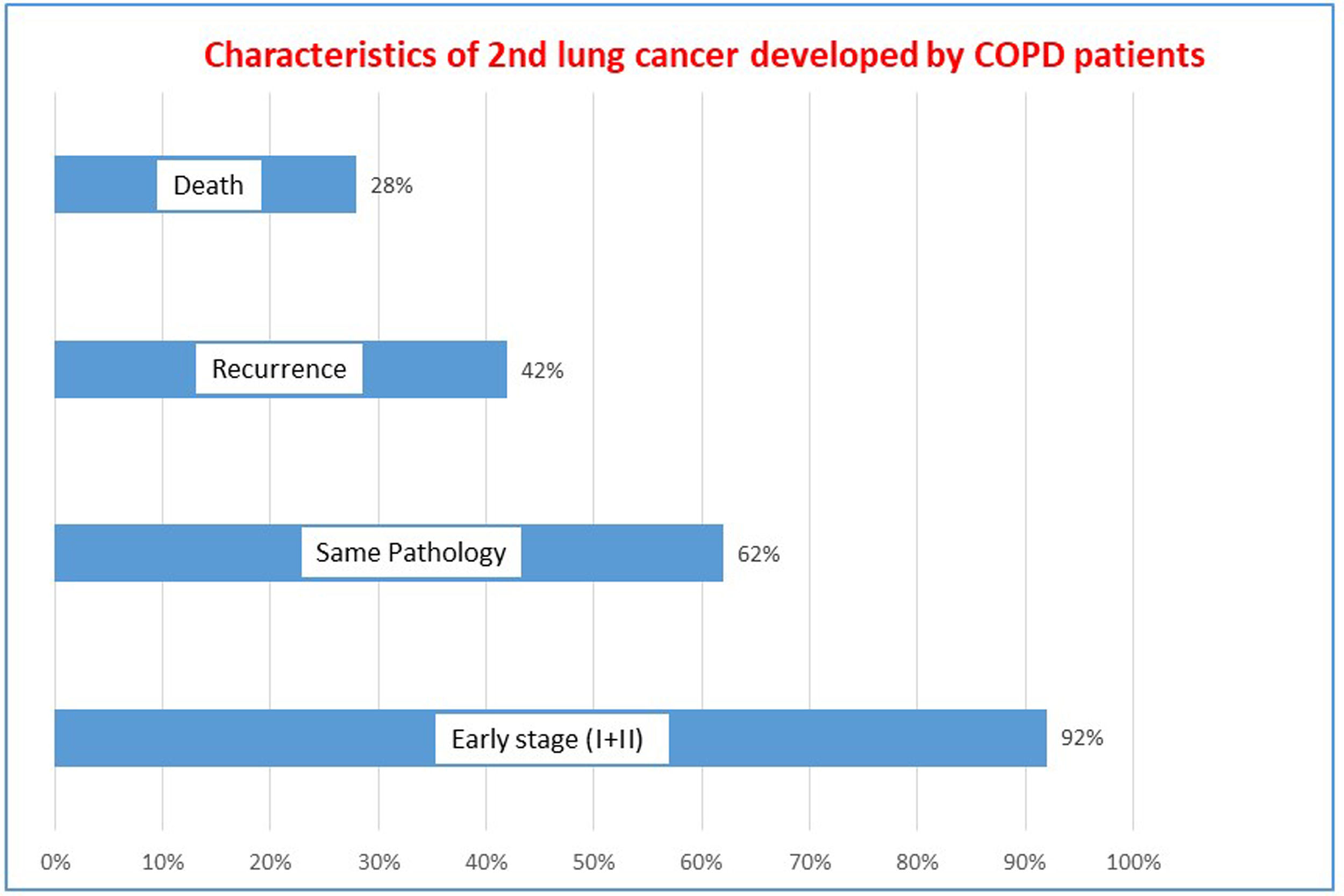
Fourteen out of the 72 COPD patients diagnosed with LC, developed either recurrence or a metachronous tumor during follow up. Eight experienced a probable recurrence (defined as a tumor of identical histology diagnosed within 5 years of follow up) and 6 a metachronous tumor. The most frequent LC histology was adenocarcinoma (79%) followed by squamous cell carcinoma (21%). The characteristics of metachronous tumors are described in Fig. 2. Most of them can be detected in early stages, and half were of the same histologic type as the previous tumor. Mortality was low with a median survival>10 years.
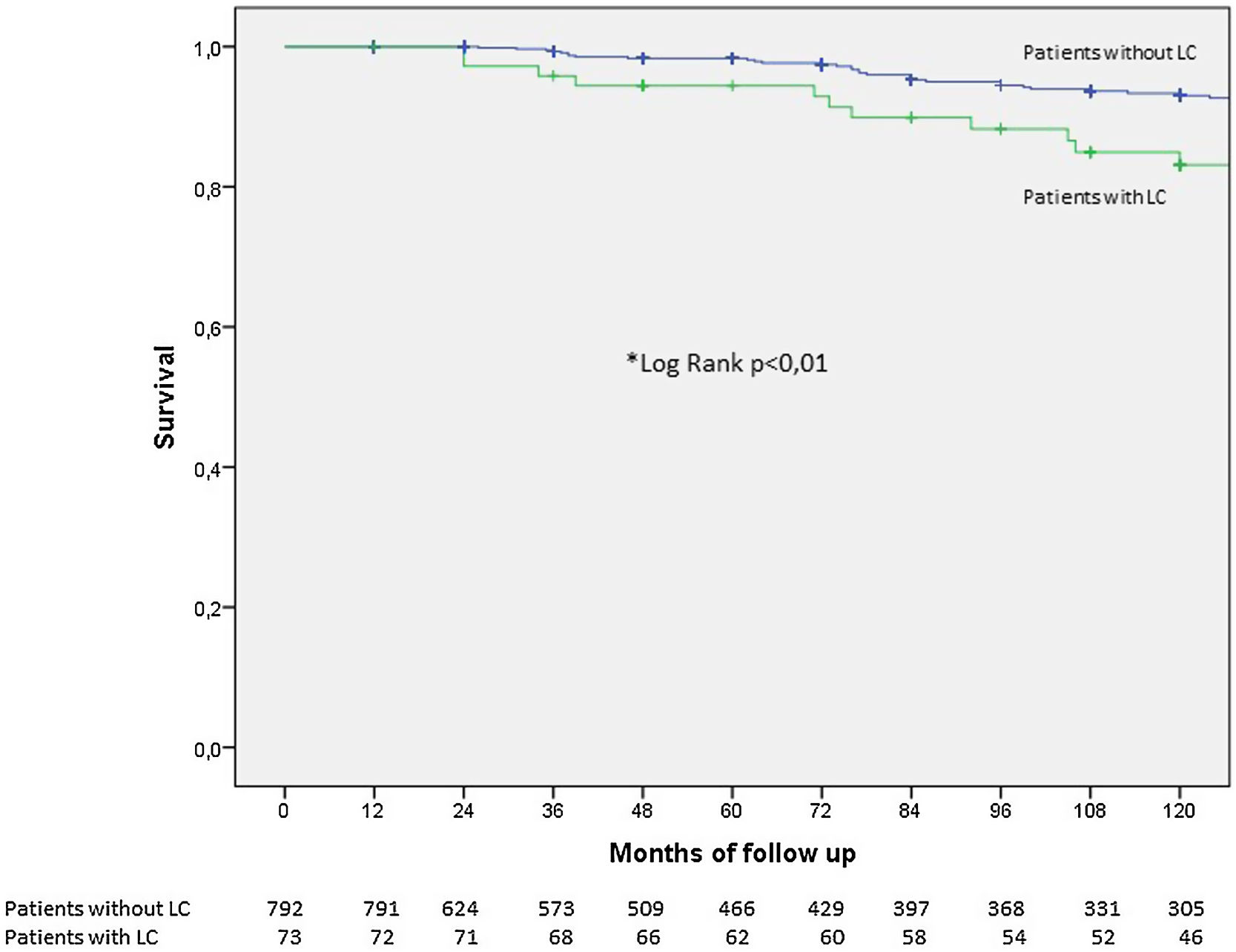
Fig. 3 shows the Kaplan–Meier survival curves for COPD patients with and without LC. As expected, those with LC had worse survival, albeit 82% of them were still alive at 10 years.
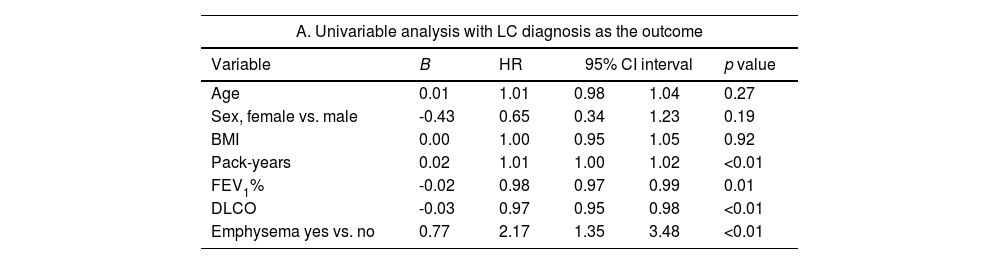
When we explored the factors associated with an LC diagnosis in this selected sample of COPD patients (see Table 4), smoking history, degree of airflow obstruction and emphysema remained significant on multivariate analysis.
Uni and multivariable analysis exploring the factors associated with LC diagnosis.
| A. Univariable analysis with LC diagnosis as the outcome | |||||
|---|---|---|---|---|---|
| Variable | B | HR | 95% CI interval | p value | |
| Age | 0.01 | 1.01 | 0.98 | 1.04 | 0.27 |
| Sex, female vs. male | -0.43 | 0.65 | 0.34 | 1.23 | 0.19 |
| BMI | 0.00 | 1.00 | 0.95 | 1.05 | 0.92 |
| Pack-years | 0.02 | 1.01 | 1.00 | 1.02 | <0.01 |
| FEV1% | -0.02 | 0.98 | 0.97 | 0.99 | 0.01 |
| DLCO | -0.03 | 0.97 | 0.95 | 0.98 | <0.01 |
| Emphysema yes vs. no | 0.77 | 2.17 | 1.35 | 3.48 | <0.01 |
| B. Multivariable analysis with LC diagnosis as the outcome | |||||
|---|---|---|---|---|---|
| Variable | B | HR | 95% CI interval | p value | |
| Pack-years | 0.01 | 1.01 | 1.00 | 1.03 | 0.01 |
| FEV1% | -0.02 | 0.97 | 0.96 | 0.99 | <0.01 |
| Emphysema yes vs. no | 0.85 | 2.34 | 1.25 | 4.37 | <0.01 |
Abbreviations: BMI: body mass index, FEV1: forced expiratory volume in first second, DLCO: diffusion capacity for carbon monoxide.
The present observational study of COPD patients screened for a prolonged period of time with LDCT, confirms that LC can be detected early stages with excellent long-term survival in these high-risk patients compatible with curative state. This is one of the first studies to specifically analyze lung cancer screening outcomes in patients with COPD.
Current GOLD guidelines recommend screening COPD patients who meet the inclusion criteria of randomized trials proving the benefit of screening for LC with LDCT.11,12 This important recommendation poses several challenges in routine clinical practice for patients who have altered lung function, competing causes of death, and more aggressive tumors.6 Physicians caring for these patients must consider these challenges when engaging in shared decision making balancing the risks and benefits of screening in the context of COPD.3,4,13,14 The present study demonstrates that LC can be cured if detected early in this challenging cohort.
Our results also confirm that the prevalence and incidence of LC in screened COPD patients is higher than previously reported for screening cohorts without spirometry. Lung cancer prevalence (baseline screening LDCT) in our COPD cohort (2.8%) tripled the prevalence reported by the NLST (1.0%) and NELSON (0.9%) investigators.11,12 Similarly, the incidence of LC detected during subsequent rounds of screening in patients with spirometry proven COPD (annual detection rate in the follow up rounds of screening) was greater in our cohort (annual rate of 0.68% vs. 0.24% in the NLST and 0.41% in the NELSON studies).11,12 The prevalence and annual detection rate of LC in our COPD cohort compared favorably with the only information available on LCS in COPD patients obtained from the ACRIN arm of the NLST study8 (Prevalence of 2.8% vs 1.28% and annual detection rates of 0.68 vs. 0.46%). This highlights the importance of airflow limitation and the emphysema phenotype in the context of screening. Arguably, both altered lung function and emphysema may be considered potential biomarkers of LC risk.3,4,9 The higher prevalence of emphysema in our cohort (50%) might explain the greater combined prevalence and incidence of LC in our program when compared to the NLST, which reported a 30% prevalence of emphysema.15
In order for screening to succeed as secondary prevention strategy, the intervention must lead to a stage shift in LC. Our study suggests that this is possible in a selected cohort of COPD patients, with 74% of LC diagnosed in early stages (92% of the LC from the prevalence screening, 79% of the LC from the incidence screening) as shown in Table 3. This finding also applies to those diagnosed with metachronous second primaries (92% diagnosed in early stages of disease) (Fig. 2). This is an important finding that has implications for survival and cost-benefit analysis when compared to previous randomized control trials11,12 (early stage % in prevalence screening: NLST 89%–NELSON 64%, incidence screening: NLST 57%–NELSON 74%). Data from a retrospective analysis from the ACRIN arm of the NSLT cohort, suggested that COPD patients have more aggressive LC.6 Although there are some differences in the COPD population studied in the ACRIN arm of the NLST (20% of patients with GOLD spirometric stages 3–4 vs. 7% in this study with advanced COPD), our study suggests that LDCT based screening results in a stage shift, notwithstanding tumor aggressiveness.
The distribution of LC histology was similar in our cohort when compared to that reported in other LCSP and specifically in GOLD 1–2 COPD patients from the ACRIN arm of the NLST study.6 Adenocarcinoma was the most common histology, followed by squamous cell carcinoma and small cell lung cancer.
Another important finding from the present study is that long term follow up results in metachronous primaries in some patients with COPD. In our cohort, 20% of patients developed a second tumor during follow up (either a recurrence or a new primary LC). This is probably a consequence of combined efficacy and long term follow up available for this cohort of COPD patients who lived long enough to develop multiple tumors. Most of these (60%) were diagnosed more than 5 years after the original tumor was resected, suggesting a new LC (rather than a recurrence). Half of those were clearly metachronous tumors with different histology, but most importantly, 92% were diagnosed in early stages and therefore curable (mean survival of 148±52 months). This finding represents a paradigm shift in the epidemiology of COPD and LC. Historically, an LC diagnosis in the context of COPD was a death sentence.16 This paradigm shift should be taken into consideration when evaluating treatment options for COPD patients and informing them of the benefits and harms of screening in the context of shared decision-making. Furthermore, because many of these patients have limited functional reserve, treatment options capable of preserving lung function (FEV1 and DLCO), should be contemplated in the context of screening COPD patients.
The association of COPD and LC has been well described, and factors associated with LC diagnosis in this selected sample of COPD patients were similar to those previously described in the literature.3,4,9 Cumulative tobacco exposure and FEV1% were significant risk factors on multivariate analysis.8 The presence of emphysema was also independently associated with a hazard ratio of 2.34. As previously reported in several studies in COPD patients, this imaging-based biomarker could be used to select the best candidates to participate in LCSP.9 Individuals with mild to moderate COPD and emphysema are arguably among the best candidates for screening.
Obvious strengths of the present study include long term follow up with annual screening (8.5 years) and post-bronchodilator spirometry. However, the study has several limitations. Our COPD patients were predominantly male, so generalizability of our results to female COPD screening candidates is limited. Studies in female COPD patients with characteristics similar to the one included in this study should be undertaken in order to confirm the greater benefit found in other LCSP.12 Improving participation of female patients in screening is one of the goals of the SOLACE consortium funded by EU4Health funds in which our center participates.17 Secondly, according to the GOLD guidelines definition, COPD is defined by the presence of a post bronchodilator FEV1/FVC<0.70 with risk factors and symptoms. Unfortunately, we did not register how many of these patients had symptoms compatible with the disease such as dyspnea, chronic cough, sputum production, wheezing or frequent respiratory infections. That notwithstanding, most of the studies published in the literature reporting on the association between COPD and LC failed to consider such symptoms. Thirdly, we did not have a control arm in our study of COPD patients who were not screened. Randomized prospective studies are needed to further improve our understanding of the promising benefits accrued by screening patients with COPD. Unfortunately, the present analysis only included mainly people with COPD with mild to moderate degree of AO therefore the potential impact of this intervention in patients with other (GOLD spirometric stages 3 and 4) degrees of AO could not be explored. As previously stated, the available data suggests8 that screening in these patients is not recommended. Lastly, we had a significant loss of follow up in our cohort. This could be explained by the fact that some participants did not want to continue in the program or by the fact that this is a program performed in a private institution where the insurance companies or the patient itself has to paid for the screening. Nevertheless, the mean follow up of the patients was 8.5 years.
InterpretationThis long-term study of LCS in a selected cohort of mainly male, mild to moderate people with COPD, demonstrated that screening allowed the diagnosis of LC in early stages with survival compatible with curative state.
ContributionsPlanning: JPdT, JZ, LS, GB. Conduction: JPdT, AC, ABA, JZ, GB, AE, MM, DM, MR, MMO, CF, JP, DL, LM, JB, MTPW, MdF, LS. JPdeT is the guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article.
Data sharingData use in the analysis is available upon request.
FundingThis project is co-funded under the EU4Health Programme 2021–2027 under grant agreement no. 101101187.
Conflict of interestsNo conflicts to declare from all authors.