The application of positive expiratory pressure (PEP) devices during exercise had been proposed in order to counteract the pulmonary hyperinflation, reduce the dyspnea and thus increase the exercise tolerance in patients with severe chronic obstructive pulmonary disease (COPD). This randomized controlled crossover trial investigated the effect of two different levels of PEP (1cmH2O and 10cmH2O) on distance covered at 6min walk test (6MWT) in patients with severe COPD. Secondary outcomes were the evaluation of PEP effects on physiological and pulmonary function variables.
MethodsSeventy-two severe COPD patients, referred to our hospitals as in and out patients, were recruited. A basal 6MWT without devices was performed on the first day, and then repeated with PEP 1cmH2O (PEP1) and 10cmH2O (PEP10), with a randomized crossover design. Slow and forced spirometries, including the inspiratory capacity measure, were repeated before and after each 6MWT.
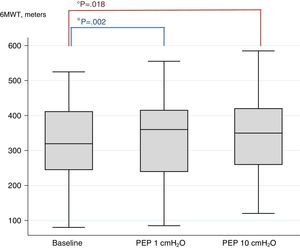
Results50 patients (average age 69.92 year, mean FEV1 41.42% of predicted) concluded the trial. The 6MWT improved significantly among both PEP levels and baseline (323.8mt at baseline vs 337.8 PEP1 and 341.8 PEP10; P<.002 and P<.018, respectively). The difference between PEP10 and PEP1 did not reach the significance. No improvements were found in pulmonary function, symptoms and physiological variables after the 6MWT.
ConclusionsIn patients with severe COPD, the application of 1cmH2O of PEP seems to improve the exercise tolerance as 10cmH2O, with similar dyspnea. Further studies should investigate the effects of low levels of PEP on aerobic training programs.
La aplicación de dispositivos de presión espiratoria positiva (PEP) durante el ejercicio se había propuesto con el objetivo de contrarrestar la hiperinflación pulmonar, reducir la disnea y mejorar así la tolerancia al ejercicio de los pacientes con enfermedad pulmonar obstructiva crónica (EPOC) grave. En este ensayo controlado, aleatorizado y cruzado se investigó el efecto de dos niveles de PEP (1cm de H2O y 10cm de H2O) sobre la distancia recorrida por pacientes con EPOC grave durante la prueba de marcha de 6min (PM6M). Los criterios de valoración secundarios fueron los efectos de la PEP sobre variables fisiológicas y de la función pulmonar.
MétodosSe reclutaron setenta y dos pacientes con EPOC grave, hospitalizados o ambulatorios, derivados a nuestros hospitales. El primer día de este estudio aleatorizado y cruzado se efectuó la PM6M sin ningún dispositivo, y posteriormente se repitió con una PEP de 1cm de H2O (PEP1) y de 10cm de H2O (PEP10). Antes y después de cada PM6M se practicaron espirometrías lentas y forzadas, que incluyeron la medición de la capacidad inspiratoria.
ResultadosCincuenta pacientes (edad media 69,92 años, FEV1 medio 41,42% del previsto) finalizaron el ensayo. La PM6M mejoró significativamente con los dos niveles de PEP, en comparación con la situación inicial (323,8m al inicio vs 337,8 con la PEP1 y 341,8 con la PEP10; p<0,002 y p<0,018, respectivamente). La diferencia entre la PEP10 y la PEP1 no fue significativa. No se observaron mejorías en la función pulmonar, los síntomas ni las variables fisiológicas posteriores a la prueba PM6M.
ConclusionesEn pacientes con EPOC grave, la aplicación de una PEP de 1cm de H2O parece mejorar la tolerancia al ejercicio, al igual que lo hace la aplicación de 10cm H2O y con un grado similar de disnea. Deben realizarse otros estudios para investigar los efectos de los niveles bajos de PEP en los programas de entrenamiento aeróbico.
In patients with chronic obstructive pulmonary disease (COPD), exercise training is a core component of pulmonary rehabilitation, supported by high-level evidence.1 The 6-min walk test (6MWT) has been adopted as standard procedure for exercise capacity assessment in patients with pulmonary diseases.2 It is a simple, widely used and reliable test, useful for evaluating the functional status and prognosis of patients with a wide variety of diseases and the effects of several interventions, such as rehabilitation, pharmacological therapy and oxygen supplementation.3–5
Patients with COPD tend to stop exercising because of dyspnea rather than leg fatigue.5,6 Their physical performance may be reduced by a significant ventilatory limitation to exercise, particularly if peripheral muscle strength is not impaired.7–9 Consequently, adjunctive strategies to improve exercise tolerance have been proposed, such as non-invasive ventilation10 and the application of positive expiratory pressure (PEP).11
PEP is applied at the mouth in order to counterbalance the dynamic compression of the airways that leads to exercise-induced pulmonary hyperinflation. In some patients with COPD, reduced elastic recoil and the presence of expiratory flow limitation lead to pulmonary hyperinflation. Patients may present increased residual volume at rest (static hyperinflation) and/or may develop hyperinflation under strain (dynamic hyperinflation), as the increase of the respiratory rate shortens the expiratory time.12 Furthermore, airway dynamic compression may occur during expiration, especially at high flow rates. The equal pressure point (EPP) is shifted peripherally, resulting in an earlier closure of the peripheral airways with consequent air trapping. This phenomenon causes a gradual shift of the tidal volume (Vt) to higher levels of functional residual capacity (FRC) and a progressive decrease in inspiratory capacity (IC). Dynamic pulmonary hyperinflation can be quantified by measuring that decrease in IC.13
The application of PEP increases intraluminal airways pressure and moves the EPP back to the upper airways, reducing dynamic compression and subsequently limiting pulmonary hyperinflation.11,14 A similar effect is obtained by pursed lip breathing (PLB), which can be considered spontaneous PEP.15
In COPD patients, some studies investigated the effects of PEP on strength, endurance, lung volumes or walking exercise. Padkao et al.14 showed that the use of a flow-dependent conical-PEP lengthens isotonic quadriceps strength exercise periods. It also produces an increase in slow vital capacity (SVC) and in IC (+200ml, P=.05), with decreased pulmonary hyperinflation. Monteiro et al.16 applied PEP using an oronasal mask after a submaximal treadmill exercise in moderate-to-severe COPD patients, reporting an increase in IC after exercise. Furthermore, Martin and Davenport17 demonstrated that extrinsic 10cmH2O PEP reduced post-exercise dyspnea in COPD patients. More recently, in severe COPD patients, Nicolini et al.11 found that an adjunctive 5cmH2O PEP, administered via mouthpiece during the execution of a 6MWT, significantly improved the distance covered, oxygen saturation, and heart rate.
However, contrasting results were reported by Wibmer et al.,18 who applied flow-dependent PEP ranging from 10 to 20cmH2O during the 6MWT in patients with stable mild-to-severe COPD. They showed that PEP reduced FRC and the residual volume (RV) after exercise, but the PEP group walked 30m less than controls. Despite these reports, no studies have described the optimal level of PEP that could improve exercise tolerance.
In this context, the main aim of our study was to investigate the effect of two different levels of PEP valve (1cmH2O vs 10cmH2O) on the distance covered during a 6MWT (6MWD), compared to the basal 6MWT performed without PEP, in patients with severe COPD (FEV1<50%). Secondary outcomes were the evaluation of PEP effects on pulmonary function, symptoms and vital parameters immediately after the 6MWT.
MethodsThis prospective, single-blind, randomized crossover study was performed at the Respiratory Rehabilitation Unit of Sestri Levante Hospital, Italy and the Pulmonary Rehabilitation Unit of Fondazione S. Maugeri IRCCS, Lumezzane, Brescia Italy, from January 2013 to October 2013.
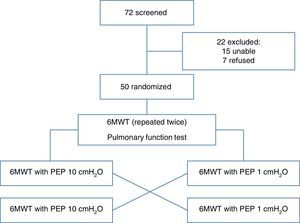
PatientsWe recruited 72 consecutive severe COPD subjects (FEV1<50%) referred to our hospitals as in- and outpatients. The inclusion criteria were: age older than 18 years, clinical stability with no changes in medication within two weeks prior the test, and no exacerbations for at least three weeks. Exclusion criteria were: history of bronchial asthma, severe bullous emphysema with history of spontaneous pneumothorax, absence of written consent, and orthopedic and neurological diseases limiting the ability to perform a 6MWT. Fifty patients were included in the study and 22 were excluded (15 were unable to perform 6MWT and 7 did not provide written informed consent). The protocol received approval from the Institutional Ethics Committee of ASL 4 Chiavarese, Italy and was carried out according to the Declaration of Helsinki. The study was registered as ChiCTR-TTRCC-12002761 www.chi.ctr.org.
Study ProtocolAll patients underwent two baseline 6MWTs, a baseline spirometry and a whole-body plethysmography on the first day of admission to the rehabilitation facility. On the second day, we started the protocol and the subjects were randomized to two groups: Group 1 performed the 6MWT with an adjunctive PEP of 10cmH2O (PEP10) and Group 2 with an adjunctive PEP of 1cmH2O (PEP1). Each group then changed the test sequence in a crossover design.
InstrumentsPEP was used during the development of the 6MWT. It was administered by a PEP-valve (PEEP Valve, Ambu, Denmark), which consists of an adjustable threshold-load expiratory pressure valve in the range 0–20cmH2O, applied on expiration in a 2-way circuit, with a valve that opens during inspiration. The 2-way circuit is connected to an oronasal mask via a 20-mm inner diameter and 100-mm length tube. As the 100mm tube adds some degree of further resistance to patient exhalation, we calculated the total PEP applied at the mouth by including a manual manometer in the circuit. At rest, the expiratory resistance in the PEP10 group was set at 10cmH2O, while in the PEP1 group, resistance was set at 1cmH2O.
MeasurementsAll 6MWTs were performed according to the American Thoracic Society guidelines in a flat, straight, indoor corridor (30m marked by colored tape at each end to indicate turnaround points).19,20 Oxygen saturation and heart rate were recorded continuously with a lightweight Bluetooth wireless oxymeter (Avant 9600, Nonin Medical Inc., US). Respiratory rate, dyspnea (assessed on a Borg scale) and distance walked in meters were recorded at the beginning and at the end of each test. The patients rested at least 1h between each test.
All patients performed a baseline spirometry and whole-body plethysmography (VMax 20 PFT Sensor Medics Yorba Linda, CA, US), according to American and European (ATS/ERS) guidelines.21
Furthermore, functional lung tests (slow and forced spirometries) were conducted using a portable spirometer (Spirolab III, Medical International Research, Italy). Measurements were obtained immediately at the beginning and within 1min of the end of each 6MWT with PEP1 and PEP10.
Statistical AnalysisData analysis was performed with statistical software (Stata Statistical Software: Release 12. StataCorp LP. College Station, TX, US).
Primary outcome was 6MWD with different PEP levels (PEP1, PEP10). Secondary outcomes were changes in respiratory functional measurements [dyspnea assessed by Borg scale, respiratory rate (RR), pulse-oxymetry] and spirometry measurements [slow vital capacity (SVC), inspiratory capacity (IC), forced vital capacity (FVC), expiratory reserve volume (ERV)]. A statistician not involved in the study generated the randomization schedule, using a randomization table from a computer software program. The randomization assignments were provided to the recruiting physician in sealed envelopes.
Descriptive analyses are presented as mean±standard deviation. Spirometry parameters obtained in PEP1 and PEP10 tests were compared using by paired t-tests. Differences between the three conditions (baseline, PEP1, PEP10) were studied by ANOVA for repeated measures (Fisher's test). When significant differences were found, post-hoc analysis with paired t-test and Bonferroni correction was done.
The investigators who performed the study data analysis were blinded to patient assignments.
ResultsSeventy-two patients were initially recruited. Fifteen subjects were excluded because they were unable to perform the 6MWT and 7 subjects refused to participate, so the final sample comprised 50 subjects. Table 1 describes the clinical and anthropometrical characteristics of the study patients. The patients were relatively elderly, predominantly male, and had severe COPD. A quarter of them were receiving long-term oxygen therapy due to associated respiratory failure. All patients had also severe static pulmonary hyperinflation. All 50 patients completed the study. Patient disposition is described in Fig. 1.
Anthropometrics and Baseline Characteristics of Patients Studied.
| Variable | Patients (n=50) | |
|---|---|---|
| Mean | SD | |
| Sex (male), % | 69.9 | 7.3 |
| GOLD level, % | ||
| III | 73.5 | |
| IV | 24.5 | |
| Long term oxygen therapy, % | 24.5 | |
| Age, years | 69.9 | 7.3 |
| BMI | 26.7 | 6.3 |
| FVC, %pred | 69.56 | 15.83 |
| FVC, L | 2.13 | 0.73 |
| SVC, L | 2.21 | 0.76 |
| FEV1, %pred | 41.43 | 12.54 |
| FEV1, L | 1.03 | 0.56 |
| FEV1/FVC | 49.43 | 14.63 |
| TLC, %pred | 114.71 | 27.27 |
| IC, L | 1.59 | 0.54 |
| ERV, L | 0.78 | 0.49 |
| RV, %pred | 181.67 | 58.10 |
| SpO2% | 94.14 | 2.33 |
| DLCO(% pred) | 63.64 | 12.83 |
| Basal dyspnea, Borg score | 1.65 | 1.93 |
BMI: body mass index; FVC: forced vital capacity; FEV1: forced expiratory volume at the 1st second; TLC: total lung capacity; IC: inspiratory capacity; ERV: expiratory reserve volume; RV: residual volume; SpO2: peripheral oxygen saturation; DLCO: carbon monoxide diffusion capacity.
F-test for primary outcome (6MWD) shows a significant difference in results among different PEP levels and baseline (F-test 0.02). Post-hoc analysis shows that the use of PEP1 and PEP10 produced an increase in walking distance (PEP1 P<.002 and PEP10 P<.018), compared to baseline. There was no significant difference between PEP1 and PEP10. Fig. 2 shows the box plots of 6MWD.
All changes (end of 6MWT−baseline) in secondary outcomes are reported in Table 2. We observed a tendency to decreased HR and increased RR in both PEP1 and PEP10 condition, compared to baseline, which did not reach statistical significance. No significant difference was found between PEP1 and PEP10 in changes in pulmonary function, symptoms and physiological variables.
Changes in Secondary Outcomes Among Different PEP Settings, Mean (SD).
| No PEP | PEP1 | PEP10 | P Value | |
|---|---|---|---|---|
| Final dyspnea, BORG scale | 5.37 (2.32) | 5.18 (2.29) | 5.65 (2.43) | .61 |
| Δdyspnea, BORG scale | 3.91 (2.22) | 3.86 (2) | 3.47 (2.04) | .52 |
| Δdyspnea% | 176 (172) | 244 (228) | 161 (157) | .23 |
| Final leg fatigue, BORG scale | 5.43 (1.36) | 4.90 (1.56) | 5.62 (1.22) | .17 |
| Δleg fatigue, BORG scale | 3.05 (1.16) | 2.79 (0.82) | 2.95 (0.86) | .67 |
| Δleg fatigue% | 172 (145) | 188 (163) | 205 (95) | .75 |
| Final RR, acts/min | 25.75 (4.44) | 25.53 (5.53) | 25.88 (7.74) | .94 |
| ΔRR, acts/min | 5.46 (3.87) | 4.37 (3.47) | 3.86 (3.66) | .06 |
| RR, % of change | 28.7 (19.9) | 23.5 (22.7) | 19.3 (18.8) | .10 |
| Final HR, beat/min | 93.53 (13.77) | 97.10 (16.77) | 97.06 (16.53) | .44 |
| ΔHR, beat/min | 18.61 (12.42) | 13.76 (9.67) | 16.30 (12.58) | .07 |
| HR, % change | 25.4 (18.9) | 18.6 (15.5) | 21.2 (16.8) | .15 |
| Final SpO2, % | 90.55 (5.66) | 91.82 (4.10) | 90.78 (6.81) | .50 |
| ΔSpO2, % | −3.33 (6.25) | −3.45 (4.96) | −2.49 (3.89) | .60 |
| SpO2, % change | −3.53 (6.68) | −3.67 (5.41) | −2.62 (4.15) | .60 |
| ΔIC, L | 0.03 (0.21) | 0.04 (0.27) | .90 | |
| IC, % change | 1.27 (15.72) | 3.42 (19.37) | .56 | |
| ΔSVC, L | 0.08 (0.23) | 0.10 (0.51) | .82 | |
| SVC, % change | 2.87 (12.56) | 8.08 (37.11) | .39 | |
| ΔERV, L | −0.02 (0.28) | −0.06 (0.28) | .50 | |
| ERV, % change | −15.91 (85.33) | −30.04 (159.64) | .60 | |
| ΔFVC, L | 0.08 (0.19) | 0.15 (0.52) | .40 | |
| FVC, % change | 4.47 (10.15) | 11.02 (44.94) | .34 |
RR: respiratory rate; HR: heart rate; IC: inspiratory capacity; SVC: slow vital capacity; ERV: expiratory residual volume; FVC: forced vital capacity. The percentage of change has been calculated as Δ*100/basal value (at rest before starting the 6MWT).
A reduction in IC of 10% and/or >150ml, compared to baseline was defined as dynamic hyperinflation.22,23 Ten subjects showed dynamic hyperinflation and 40 did not. In the 40 subjects without dynamic hyperinflation, we observed a significant change in the distance covered (Δ meters) if 1cmH2O PEP was applied. No significant change was found if 10cmH2O was applied. These results appear in Table 3.
Results in Covered Distance (6MWD) for Patients With or Without Dynamic Hyperinflation.
| PEP1 | PEP10 | |||||
|---|---|---|---|---|---|---|
| Patients | Meters | Δ Meters | Patients | Meters | Δ Meters | |
| Patients with dynamic hyperinflation | 10 (20%) | 290 (95) | −3.22 (21.98) | 8 (16.0%) | 304 (132) | 14.42 (31.08) |
| Patients with no dynamic hyperinflation | 40 (80%) | 342 (124) | 20.83 (26.71) | 42 (84.0%) | 345 (110) | 24.39 (31.91) |
| P-value | .24 | .001 | .38 | .45 | ||
6MWD: 6-min walk distance; PEP: positive expiratory pressure.
In this randomized crossover trial, conducted in patients with severe COPD, we found that the application of 1cmH2O PEP during the execution of 6MWT can improve the distance walked to the same extent as the application of 10cmH2O PEP, without affecting the cardiovascular response to exercise. We did not find any effect of PEP on dyspnea at the end of exercise and post-exertion lung dynamic volumes, particularly IC, SVC, FVC and ERV. Moreover, we found that the improvement of 6MWD is greater in patients with no or low dynamic hyperinflation when a low PEP is applied.
So far, only two other crossover randomized trials have investigated the effects of a PEP device on 6MWD, with contrasting results.
In 2013, Nicolini et al.11 found that a threshold PEP of 5cmH2O improved the distance walked during the 6MWT in patients with moderate-to-severe COPD. They also demonstrated a significant improvement in post-exercise oxygen saturation and a decrease in peak heart rate during the 6MWT with PEP, compared to controls without devices. However, in the same year, Wibmer et al.18 investigated the same kind of patients and found that, with a flow-dependent PEP in the range of 10–20cmH2O, patients walked 30.8m less than the controls without devices (352±92m with no PEP, 321±93m with PEP, P=.001). In their study, they demonstrated the efficacy of flow-dependent PEP on lung dynamic volumes, as total lung capacity, FRC and RV were significantly reduced after the 6MWT with PEP, compared to control group.
Although the three studies used different kinds of PEP, it seems that the application of lower pressures produces better results in terms of distance covered during the 6MWT. However, the “right” level of PEP during exercise is still largely understudied.
In this context, Martin et al.17 applied two levels of threshold PEP (13.8 and 2cmH2O) and demonstrated a significant decrease in dyspnea with the use of the higher level of PEP after a treadmill constant load test. However, these results cannot be compared with ours, because the authors applied PEP only after the conclusion of the exercise test, rather than during effort.
Recently, Bhatt et al.15 reported good results regarding exercise tolerance improvement in patients with severe COPD. They conducted a crossover trial investigating the effects of PLB on 6MWD and found that patients walked 34.9m more if they breathed with volitional PLB, compared to breathing normally (P=.002), with a concomitant reduction in RR after the test. Furthermore, they investigated the diaphragm excursion with ultrasonography and found a correlation between the improvements in 6MWD and the increase of diaphragm excursion with PLB. The authors explain their findings by suggesting that the PLB imposes a control and slows the RR, breaking the vicious circle of air trapping and dynamic pulmonary hyperinflation. This might translate into less neuro-ventilatory dissociation and a reduced perception of dyspnea. Low pressure PEP can be expected to act in a similar way.
As regards pulmonary function, low PEP has also been proposed for airway clearance in patients with COPD and hypersecretion. Venturelli et al.24 studied a mechanical device which applies 1cmH2O PEP at the mouth. They reported a significant improvement of IC at rest when patients breathe into the PEP device, compared to controls (+19.5% and +2.2%, P=.044). However, no data are available during exercise.
Monteiro16 and Padkao14 reported improvements in dynamic and static lung volumes, we cannot confirm the effect of PEP devices on post-exercise lung volumes. As we did not perform the spirometry immediately after the basal 6MWT, we do not have data on the behavior of the IC and the other lung volumes during the 6MWT without PEP. At present, we do not know if PEP produces any improvements in lung volumes compared to no PEP. We did not find any significant difference in the behavior of IC, SVC, ERV or FVC between PEP1 and PEP10. The application of low and high levels of PEP during the 6MWT proved to have the same effect on lung volumes.
Nevertheless, in our “ad hoc” analysis, we found that the application of low level PEP produces a greater improvement in the distance covered in the group of patients with no dynamic hyperinflation, compared to the group of patients who presented dynamic hyperinflation. This was in line with the findings of Callens et al., who observed that change in IC after walking does not correlate with 6MWD.25
In conclusion, it is difficult to compare the effects of PEP on exercise tolerance in patients with COPD, because the published studies are too heterogeneous, and use a variety of exercise protocols, types and levels of PEP and outcome measures. In order to clarify the effects of PEP on lung mechanics, it would be useful to evaluate the effect of PEP during a standardized exercise capacity test, such as the cardiopulmonary exercise test. As already discussed by Wibmer et al.,18 a better standardized evaluation of dynamic lung volumes, including at least some subsequent IC measures during the exercise, should also be carried out.
Future studies should lead to a more practical utilization of PEP devices. The effects of low level PEP during aerobic and strength training protocols, in the mid to long term, merit investigation.
Limitations of the StudySome limitations in the study were detected. Firstly, pulmonary function was not assessed directly during the test; instead there was an average delay of 1min between the end of 6MWT and the execution of the spirometry. This could have led to an underestimation of the effects of PEP on lung function.
Furthermore, two kinds of PEP devices were proposed: flow-dependent or threshold-load.26 We used a threshold-load PEP valve, but studies comparing both devices have not yet been published.
In 2012, Monteiro et al.16 demonstrated in a controlled non-randomized trial that breathing in a PEP mask at 10cmH2O during a treadmill test lasting 20min produces less dynamic pulmonary hyperinflation, compared to controls without PEP, in severe COPD patients. They demonstrated a significant increase in IC and less IC decrease after exercise with PEP, compared to the control group. In their protocol, they started the exercise with 10cmH2O PEP and, if this was not tolerated by the patient, they reduced resistance gradually to as low as 5cmH2O. They found that the mean tolerated PEP value was 8±1.5cmH2O. We did not define the highest PEP value tolerated by the patients, since PEP values higher than tolerated could limit the ability to exhale, worsening dynamic pulmonary hyperinflation, causing dyspnea and leading to poor results.
Another limitation may be related to the type of test employed. During the 6MWT, patients are allowed to slow down or stop if needed, for example in case of severe dyspnea. Moreover, the patients choose the walking speed themselves. For these reasons, it is possible that dynamic hyperinflation is not addressed during the 6MWT.
We also defined severe COPD as an inclusion criterion. If we had included patients who only had reduced IC during exercise, we may have found higher levels of significance.
Finally, the minimum clinically important difference of the 6MWD has been previously established as 25m27,28 and this threshold was not reached in our study. This could be due to the wide variability of the distance covered by the patients. However, even a small improvement obtained by having the patient breathe into a simple device, while not clinically relevant, may still indicate a correct strategy which merits further investigation.
Furthermore, a sample size analysis was not carried out and it is possible that the number of patients may have been too small to obtain more significant results.
ConclusionsIn patients with severe COPD, the application of 1cmH2O PEP seems to improve the exercise tolerance to the same extent as 10cmH2O, with similar reported dyspnea. No effects were found on lung volumes after exercise. As previously reported protocols and levels and types of PEP vary widely, further studies are recommended to confirm our results and to clarify the mechanism of action on lung mechanics. Future studies should also investigate the effects of low levels of PEP on aerobic and strength training programs.
| Authors | Year | Aim | Study type | Patients | Protocol | Primary outcome | PEP type | Results |
|---|---|---|---|---|---|---|---|---|
| Nicolini et al.11 | 2013 | PEP utilization improves 6MWD? | Prospective, randomized controlled, single blind | 100 (50 controls) in-outpatients with moderate- to-severe COPD (%FEV1 51–49%, %RV 148–140%, mean basal 6MWD 232–262mt). | 6MWT at assessment, repeated after 30min of rest with PEP in the intervention group, without devices in the control group. | 6MWT | 5cmH2O threshold-PEP connected to mouthpiece with a tube (20mm diameter, 100mm length). | PEP improves 6MWD (+61 vs +3mt, P<0.001). During 6MWT, PEP improves SpO2 and decreases FC (p<0.03); NS reduction of Borg Dyspnea and Respiratory Rate. |
| Wibmer et al.15 | 2013 | Feasibility of a nasal PEP application during exercise, effects on dynamic hyperinflation. | Randomized, crossover. | 20 mild-moderate COPD (FEV1>50%). | 6MWT repeated twice (2–24h of rest), with and without nasal PEP (crossover design), with subsequent spirometry and Borg dyspnea assessment. | 6MWD, spirometry | Nasal mask with headgear, flow-dependent PEP 10–20cmH2O. | PEP decreases TLC, FRC and RV (P<.05), but produces also a significant reduction of 6MWD (−30.8mt, P=.001). |
| Martin et al.8 | 2011 | PEP utilization reduces post-exercise dyspnea? | Double blind, crossover. | 8 outpatients (4 controls) with COPD (FEV1<50%) and habitual use of pursed lip breathing. | Treadmill test repeated twice (crossover design). At the end of the test, the intervention group performed 6 breaths in threshold PEP, the control group in a sham-PEP. | Borg dyspnea | 10cmH2O threshold PEP (13.8cmH2O if measured at the mouth) vs 2cmH2O sham-PEP. | PEP reduces dyspnea 10min after the exercise (post-exercise Borg dyspnea: 2.6 and 1.8 point, control and intervention group respectively, P<.0001). |
| Padkao et al.11 | 2010 | Conical-PEP reduces hyperinflation and increases exercise duration? | Randomized, crossover. | 13 COPD moderate-to-severe (FEV1 61%). | Leg extension at 30% 1RM with ankle weights, 15 repetitions per min per leg, with and without PEP (crossover design). | Spirometry (TLC, IC, slow VC) dyspnea, legs effort, exercise duration. | Flow-dependent conical-PEP 4–20cmH2O vs normal breathe. | PEP produces an increase of IC and slow VC (+200ml, P=.05), of the exercise duration (+107s, P=.05), and a reduction of RR during exercise (−6.1breath/min, P=.05), no adverse effects. |
| Monteiro et al.14 | 2012 | Effect of PEP on inspiratory capacity. | Non-randomized, controlled. | 17 COPD (FEV1 38%) with dynamic hyperinflation, defined as at least 15% decrease of IC after treadmill exercise. | Treadmill test lasting 20min, with subsequent IC measure. If decreased at least of 15%, the test was repeated with PEP administration (timing not specified). | IC | Oronasal mask with headgear and unidirectional valve, spring loaded PEP 5–10cmH2O. | PEP produces an increase of IC (1.45L and 1.13L with and without PEP respectively, P=.02) and less IC decrease after exercise (−0.18L and −0.57L with and without PEP respectively, P=.02). |
| Bhatt et al.19 | 2014 | Effect of pursed lip breathing on exercise capacity | Randomized, crossover | 14 COPD (FEV1 38.4%) | 6MWT at assessment, repeated with PLB | 6MWT, spirometry, MIP, MEP, diaphragmatic excursion with US | Volitional pursed lip breathing | PLB increases 6MWD (+34.9mt, P=.002), reduces RR after 6MWT (−4.4bpm, P=.003). Correlation between improvement in 6MWD and increase in diaphragmatic excursion with PLB. Greater improvement in patients with poorer baseline 6MWD. |
FEV1: forced expiratory volume 1st second; RV: residual volume; 6MWD: distance covered in 6-min walking test; TLC: total lung capacity; FRC: functional residual capacity; 1RM: 1 repetition maximum; IC: inspiratory capacity; VC: vital capacity; PLB: pursed lip breathing; MIP: maximal inspiratory pressure; MEP: maximal expiratory pressure; US: ultrasounds.
Please cite this article as: Russo D, Simonelli C, Paneroni M, Saleri M, Piroddi IMG, Cardinale F, et al. ¿Cuál es el nivel óptimo de presión espiratoria positiva (PEP) capaz de mejorar la tolerancia a la deambulación de los pacientes con EPOC grave? Arch Bronconeumol. 2016;52:354–360.