Recently, the relevant role of eosinophils as a marker of sensitivity to inhaled corticosteroids in patients with chronic obstructive pulmonary disease (COPD) has been recognized, particularly in patients with frequent exacerbations.1,2 In these subjects, peripheral eosinophils have been shown to be closely related to the rates of exacerbations3,4 and mortality5 as well as the intensity of symptomatic limitation,6 which is a major determinant of daily physical activity.7 Indeed, physical activity has become an important therapeutic target in COPD, with emerging evidence showing a relationship between sedentarism and disease progression8 and even increased mortality.9
Despite this, the information currently available about the relationship between peripheral eosinophils and daily physical activity in COPD patients is very scarce. To our knowledge, only one previous study described lower self-reported physical activity in patients with asthma-COPD overlap (ACO) than in those with other COPD phenotypes,6 although unconventional ACO criteria and a questionnaire were used to assess physical activity. Therefore, our aim was to compare the level of daily physical activity in COPD patients according to their blood eosinophil count (BEC).
We selected consecutive patients aged 40–80 years with a previous diagnosis of COPD,1 post-bronchodilator FEV1/FVC ratio<lower limit of normal, and a history of smoking (>10packs/year) who had been clinically stable in the previous 6 weeks. Exclusion criteria were: previous diagnosis of asthma or other respiratory diseases, previous treatment with systemic corticosteroids or other immunosuppressant drugs, clinical evidence of disorders associated with peripheral eosinophilia (drugs, parasitic infections, acute allergic conditions, vasculitis or hypereosinophilic syndromes), severe cardiovascular or neurological diseases, and cognitive problems or other disabling conditions that may affect physical activity. The study was performed in a single center, and it was approved by the local ethics committee, and all participants signed informed consent forms.
Data were collected for anthropometric variables, smoking history, disease duration, dyspnea level using modified Medical Research Council (mMRC) scale and Charlson comorbidity index. Post-bronchodilator spirometry was performed according to ATS/ERS recommendations10 and using Global Lung Initiative (GLI) reference values.11 A 6-min walk test was also performed following ATS guidelines, and the BODE index was calculated. A SenseWear Pro3 accelerometer (Body Media Inc, Pittsburgh, PA, USA) was placed on the patients’ non-dominant arm for 7 consecutive days to measure daily physical activity. Recordings were considered valid if they included at least 10h per day for a minimum of 4 days, including one weekend day. Physical activity level (PAL) was recorded, and patients were classified as: very sedentary (PAL<1.40), sedentary (PAL=1.40–1.69) or moderately active (PAL>1.70).12 Simultaneously, a hemogram was determined in the center's laboratory, and increased BEC was defined as 300 or more cells/μL,13 although other cut-off points were also used.
Between-group comparisons were performed using Student's t-test or chi-square tests. For comparison of physical activity levels between groups, general linear models were employed, using the presence or absence of increased BEC as a fixed factor and age, body mass index, dyspnea (mMRC), Charlson index and post-bronchodilator FEV1 as covariates. Relationship between variables was assessed with Pearson's rank correlation. The discriminative capacity of several eosinophil cut-off points to identify sedentary patients was analyzed using Receiver Operating Characteristic (ROC) curves and forward stepwise multiple logistic regression.
A total of 123 COPD patients were selected, with a mean age of 63 years, mainly men (72%) who were overweight (mean body mass index [BMI]=27.5±3.7kg/m2) and had a history of heavy smoking (47±18 pack-years). Mean time since COPD diagnosis was 9±6 years, with moderate-severe airflow limitation (post-bronchodilator FEV1 47±13% pred.), dyspnea (mean mMRC scale 2.4±1) and a mean BODE index of 4±2 points (4-year survival 67%).
The frequency of increased BEC in our sample of COPD patients was 14.6% (95%CI, 8.4–20.9%). Patients with increased BEC were slightly younger than non-eosinophilic patients (59±9 vs 64±8, P=0.026). However, both groups were homogeneous for all other anthropometric characteristics, cumulative smoking pattern and intensity, years since diagnosis, baseline dyspnea intensity, Charlson comorbidity index, severity of airflow limitation, or BODE index. No differences were identified for hemoglobin, hematocrit, or total leukocyte levels.
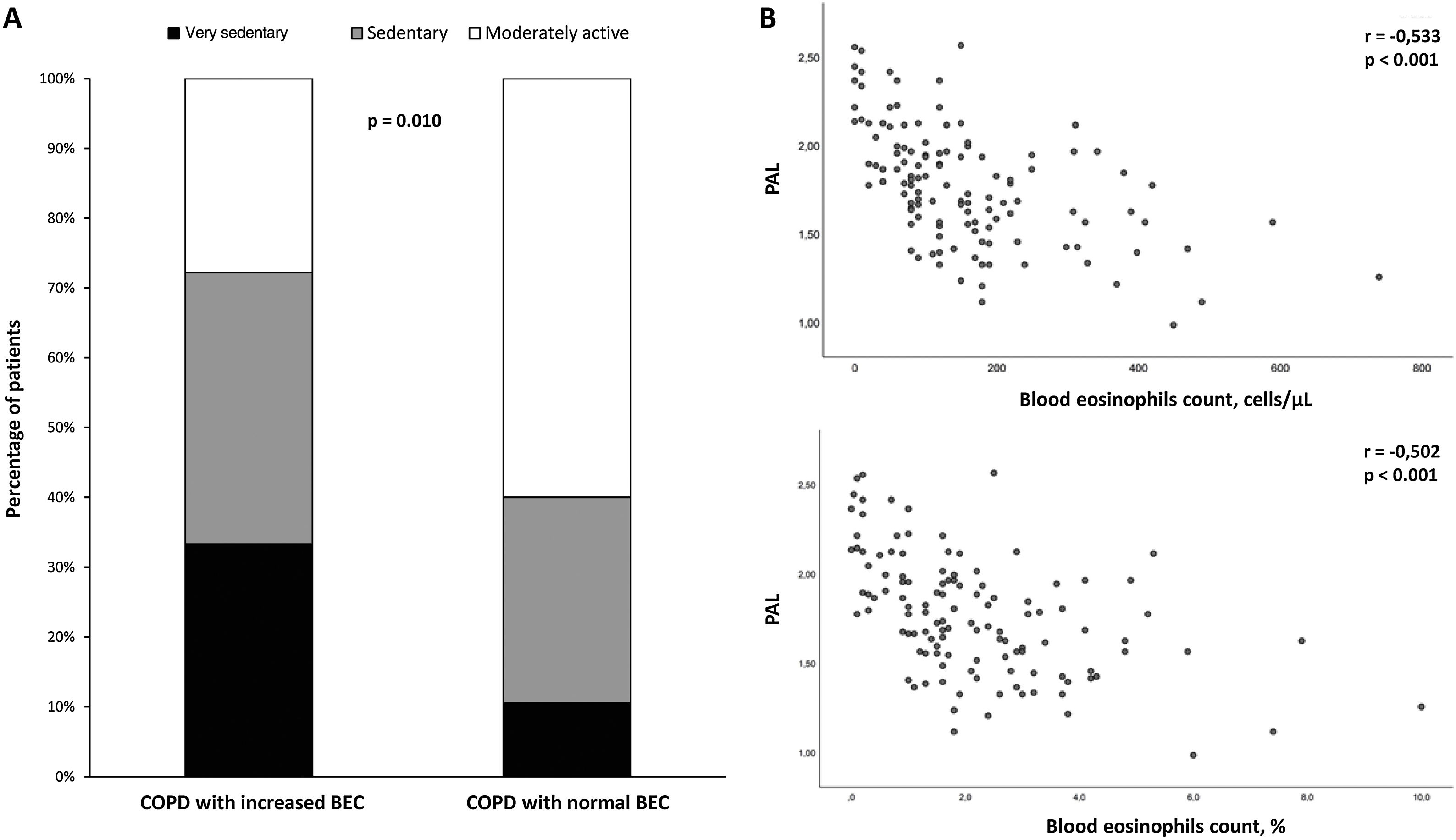
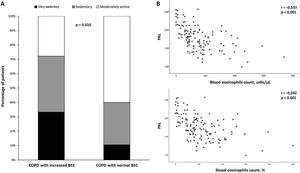
COPD patients with increased BEC had significantly lower PAL than patients with normal BEC (1.81±0.32 vs 1.54±0.31; P<0.001). This association is also reflected in the distribution of physical activity patterns of both groups (Fig. 1A). The decrease in physical activity performed by patients with COPD and increased BEC remained statistically significant after adjusting for confounding variables, such as age, BMI, dyspnea, Charlson comorbidity index and post-bronchodilator FEV1 (mean±standard error) (1.81±0.03 vs 1.53±0.08; P=0.001). In turn, an inversely proportional relationship between absolute or percentage BEC and PAL was observed (r=−0.533, P<0.001 and r=−0.502, P<0.001, respectively) (Fig. 1B).
(A) Distribution of daily physical activity patterns between COPD patients with or without increased blood eosinophils count (BEC); (B) Relationship between the number (upper panel) or percentage (lower panel) of the BEC and physical activity level (PAL) of patients with COPD. Abbreviation: r, Pearson correlation coefficient.
The discriminative capacity of several increased BEC cut-off points to identify sedentary or very sedentary patients was also assessed. The criterion of >150eosinophils/μL achieved the higher area under the ROC curve (0.71±0.05). Similarly, the multiple logistic regression model also retained a value of 150eosinophils/μL as the only cut-off point independently associated with sedentarism (adjusted odds ratio=6.2; 95%CI: 2.83–13.62; P<0.001; r2=0.227).
The prevalence of increased BEC found in our patients concurred with data from the BODE and CHAIN cohorts, which showed a prevalence of persistent eosinophilia (also defined as ≥300cells/μL) of 12.3% and 15.8%, respectively.13 In turn, different studies have correlated the intensity of increased BEC with the severity of airflow limitation. Thus, an analysis of the SPIROMICS cohort indicates that patients with ≥200eosinophils/μL have a lower FEV1, slightly increased airway wall thickness, as well as poor health-related quality of life and increased wheezing.14
There is little information about the relationship between the physical activity of COPD patients and their inflammatory response. In COPD patients without corticosteroid treatment, increased airway eosinophils have been reported after acute exercise, with a decrease in the peripheral blood eosinophil count and serum levels of proinflammatory cytokines (IL-6, IL-8 and CCL-5), suggesting an anti-inflammatory effect of exercise.15 Moreover, in older adults from the general population, genetic markers associated with increased physical activity were related to lower levels of lymphocytes and eosinophils in peripheral blood.16 Although this decrease has traditionally been considered a certain level of immunosuppression, recent evidence suggests that it might represent an increased state of immune surveillance and regulation, through the transfer of cells to peripheral tissues.17
To our knowledge, this is the first study that evaluates the relationship between the number of eosinophils in peripheral blood from COPD patients and their level of physical activity, assessed by an objective measurement. Our data show that increased BEC in COPD patients is negatively related to the level of physical activity, independently of other confounding variables such as age, severity of airflow limitation or comorbidity. However, and due to its cross-sectional design, the present study does not allow us to establish a cause-effect association for said relationship or to infer a prognostic value.