The aim of our study was to describe the incidence of infectious complications of endobronchial ultrasound guided transbronchial needle aspiration (EBUS-TBNA) and to analyze the potential risk factors in a prospective cohort of patients.
MethodsWe conducted a prospective multicenter study, with all consecutive patients referred for an EBUS-TBNA with patients at risk of developing an infectious complication (considering>10 nodal samplings, known immunosuppression, bronchial colonization and cavitated or necrotic lesions) and a second group without any risk factor.
ResultsThree hundred seventy patients were included: 245 with risk factors and 125 without risk factors (as the control group). Overall, 15 patients (4.05%) presented an acute infectious complication: fourteen in cases (5.7%) and 1 in controls (0.8%). Of these, 4 patients presented pneumonia, 1 mediastinitis, 4 obstructive pneumonitis and 6 mild complications (respiratory tract infection that resolved with antibiotic). Also 7 (1.9%) patients had self-limited fever. One-month follow-up showed 1 mediastinitis at sixteenth day post-EBUS, which required surgical treatment, and 3 pneumonias and 3 respiratory tract infections at nineteenth day (1.9%). All patients had a good evolution and there were no deaths related with infectious complication. We observed an increased risk of complication in patients with risk factors and in patients with necrosis (p=0.018).
ConclusionsThe incidence of infectious complications in a subgroup of patients with risk factors was higher than in patients without risk factors. Nevertheless, it remains low, and no fatal complication occurred, which reinforces the idea that EBUS-TBNA is a safe technique for the assessment of the mediastinum. Necrotic lesions are a risk factor of post-EBUS infection, and their puncture should be avoided.
Lung cancer is the leading cause of cancer-related death worldwide.1 In patients with lung cancer (NSCLC), accurate mediastinal lymph node staging becomes crucial to determine the prognosis of the disease and treatment choice.2 Endobronchial ultrasound guided transbronchial needle aspiration (EBUS-TBNA), is recommended as the first-choice technique for invasive mediastinal staging.3,4
EBUS-TBNA has been shown to be a safe technique, with few complications.5–8 Infectious complications including mediastinitis,9,10 pneumonia, mediastinal abscess,11,12 bacteremia13 and prolonged fever,14–17 have been reported mostly as case reports or isolated cases in retrospective studies. Although the reported incidence is low, about 0.2–0.5%8,14,18 they can be severe. For this reason, in some institutions antibiotic prophylaxis after EBUS-TBNA is administered.
Several publications on endoscopic ultrasonography (EUS) suggest avoiding puncturing cystic-type and/or necrotic lesions as risk of infectious complications19–22 but there are no clear indications and risk factor descriptions in current guidelines on EUS.23,24 Similarly, a recent retrospective study in radial endobronchial ultrasound-guided transbronchial biopsy with a guide sheath (EBUS-GS-TBB)25 and some report of cases in EBUS-TBNA suggest that sampling this sort of lesions may lead to infectious complications.6,14,26 A recent retrospective study on patients who received antibiotics recommended paying attention if the target lesion appears necrotic.18 However, in EBUS-TBNA, potential risk factors of infectious complications have not yet been validated.
The aim of our study was to describe the incidence of infectious complications of the EBUS-TBNA and to elucidate the potential determinants for infectious complications in a prospective cohort of patients with suspected risk factors.
Material and methodsDesignThis is a multicenter, prospective cohort study, coordinated by the Spanish Society of Pulmonology (PII Interventionist pulmonology 005/2015 IP). The local review board of all participant centers approved the study protocol.
The study included all consecutives patients referred for an EBUS-TBNA that presented suspected risk factors for an infectious complication and a subgroup of patients with no risk factors. They were recruited from January 2015 to September 2021. All participant centers are tertiary hospitals with a highly specialized unit in pulmonary interventionism (determined by the Spanish Society of Pulmonology).
PatientsInclusion criteria were patients over 18 years of age referred for an EBUS-TBNA, with informed consent and that presented one or more suspected risk factors: Immunosuppression; cavitated or cystic lesions; necrotic on computer tomography (CT), Positron Emission Tomography with computer tomography (PET-CT) or EBUS (at rapid on-site evaluation (ROSE) or final report); ten or more punctures during EBUS-TBNA; and chronic bronchial colonization.
Immunosuppression was defined as being on immunosuppressive treatments or chemotherapies, chronic corticoids treatment, hereditary or acquired immunosuppressive diseases such as agammaglobulinemia, human immunodeficiency virus 1 (HIV-1) positive, splenectomy, poorly controlled diabetes, or severe malnutrition.
We also included a subgroup of consecutive patients without risk factors, considered as the control group.
Patients with current febrile illness or current respiratory infection, antibiotic therapy, and impossibility of follow-up, refusal to participate or inability to sign consent, were excluded.
EBUS-TBNALinear EBUS-TBNA (BFUC180 F, Olympus Optical Co Ltd., Tokyo, Japan) was performed, under local anesthesia and sedation, using topical lidocaine spray and intravenous midazolam, propofol and fentanyl according to the usual practice of each center following standard recommendations using laryngeal mask.26 All EBUS procedures were performed by experienced (at least 10 years) pulmonologists. A 22-gauge cytology needle (NA-201SX-4022, Olympus Optical Co Ltd.) was used.
Pathological examinationThe aspirated material was placed on slides and examined in situ by an expert pathologist, using rapid hematoxylin, evaluating the viability of the sample and presence of necrosis signs (visible in the square at 10× magnification).27
Data collection and follow-up/infectious complications surveillingPatients’ characteristics including socio-demographic data, medical records (smoking history, pulmonary diseases and immunosuppression), tumor and nodal size, location, final diagnosis, presence of cavitation, mediastinal cystic lesions or necrosis signs in tumor and/or lymph nodes on CT and/or EBUS, and procedure-related characteristics including number and size of lymph nodes punctured, total number of punctures, number of EBUS stations and nodal appearance on EBUS, were collected.
Bronchial aspirate (BAS) was collected for microbiological culture. In the subgroup of patients with risk factors, thirty minutes after the EBUS-TBNA procedure we performed a blood test (with blood-count, acute phase reactants (erythrocyte sedimentation rate (ESR), C reactive protein (CRP) and Procalcitonin) and a blood culture from a peripheral vein, and axillary temperature was measured. Two hours after EBUS, a chest X-ray was performed. At the time of discharge the patient was asked to record the axillary temperature every 8h during the next 48h.
Forty-eight hours after the EBUS-TBNA procedure the patient was visited in an outpatient clinic, blood test was repeated and axillary temperature was measured. A questionnaire that included the presence/absence of fever and/or respiratory symptoms (increased volume and/or purulence of sputum, dyspnea or worsening of breathlessness) was performed. Fever was defined as an axillary temperature over 38°.
Patients were followed-up at 14, 21 and 30 days by reviewing of the medical history and by a telephone call 30 days after EBUS, collecting data about fever or any other complication.
Statistical analysisCategorical variables were described using absolute frequencies and percentages, while discrete variables were described with mean and standard deviation. Association between discrete variables was analyzed using linear regression, while associations with dichotomous variables were analyzed using logistic regressions. We used the Bonferroni method to correct for multiple testing. All analyses were conducted with version 15 of STATA (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC).
Ethical issuesThe research protocol was approved by the regional ethics committee (Ethics Committee for Clinical Research of the Hospital Germans Trias i Pujol). Reference PI-14-089.
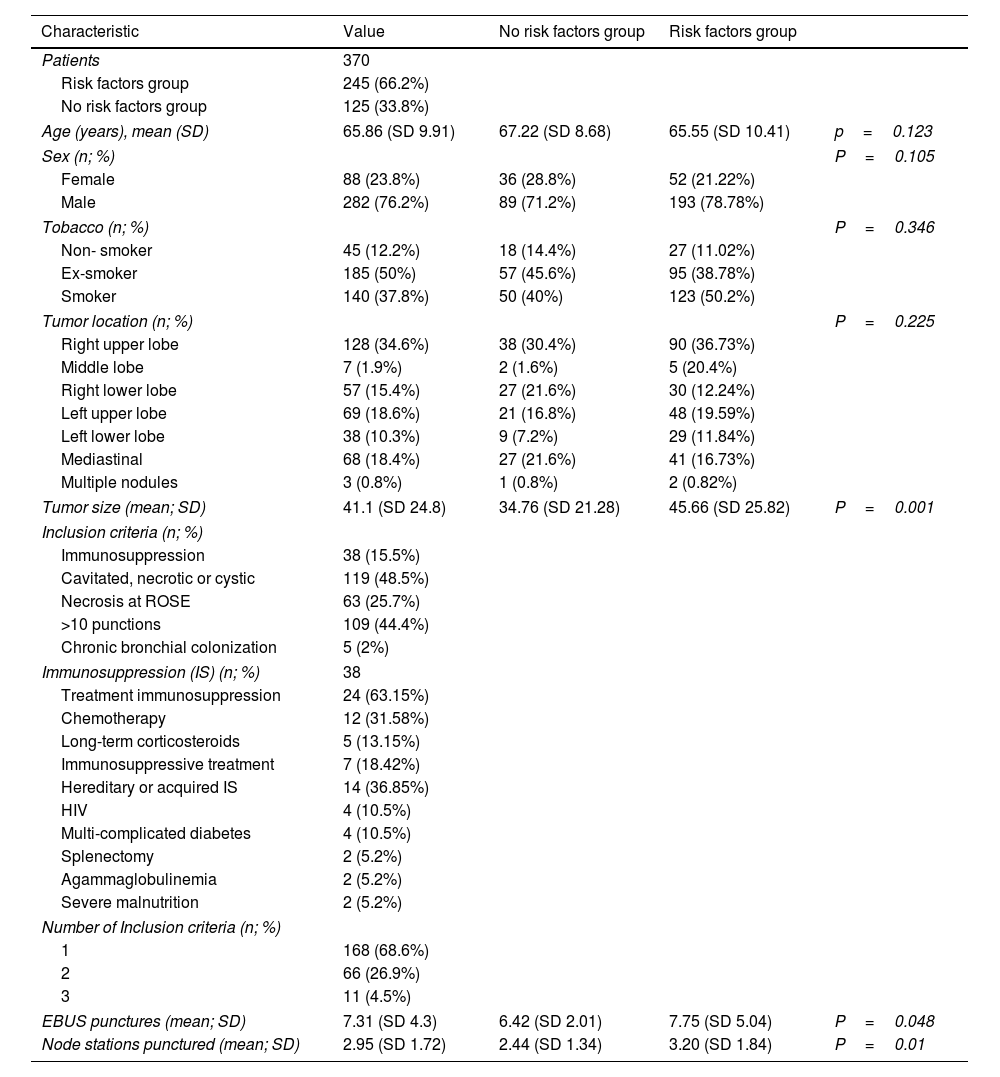
ResultsThree-hundred and seventy-five patients were recruited in three participant centers: 250 with risk factors and 125 without risk factors. Five patients were lost to follow-up and consequently excluded. Thereby 370 patients were included in the analysis: 245 with risk factors and 125 without risk factors. Patient's characteristics are described in Table 1.
Patients’ and procedure-related characteristics.
| Characteristic | Value | No risk factors group | Risk factors group | |
|---|---|---|---|---|
| Patients | 370 | |||
| Risk factors group | 245 (66.2%) | |||
| No risk factors group | 125 (33.8%) | |||
| Age (years), mean (SD) | 65.86 (SD 9.91) | 67.22 (SD 8.68) | 65.55 (SD 10.41) | p=0.123 |
| Sex (n; %) | P=0.105 | |||
| Female | 88 (23.8%) | 36 (28.8%) | 52 (21.22%) | |
| Male | 282 (76.2%) | 89 (71.2%) | 193 (78.78%) | |
| Tobacco (n; %) | P=0.346 | |||
| Non- smoker | 45 (12.2%) | 18 (14.4%) | 27 (11.02%) | |
| Ex-smoker | 185 (50%) | 57 (45.6%) | 95 (38.78%) | |
| Smoker | 140 (37.8%) | 50 (40%) | 123 (50.2%) | |
| Tumor location (n; %) | P=0.225 | |||
| Right upper lobe | 128 (34.6%) | 38 (30.4%) | 90 (36.73%) | |
| Middle lobe | 7 (1.9%) | 2 (1.6%) | 5 (20.4%) | |
| Right lower lobe | 57 (15.4%) | 27 (21.6%) | 30 (12.24%) | |
| Left upper lobe | 69 (18.6%) | 21 (16.8%) | 48 (19.59%) | |
| Left lower lobe | 38 (10.3%) | 9 (7.2%) | 29 (11.84%) | |
| Mediastinal | 68 (18.4%) | 27 (21.6%) | 41 (16.73%) | |
| Multiple nodules | 3 (0.8%) | 1 (0.8%) | 2 (0.82%) | |
| Tumor size (mean; SD) | 41.1 (SD 24.8) | 34.76 (SD 21.28) | 45.66 (SD 25.82) | P=0.001 |
| Inclusion criteria (n; %) | ||||
| Immunosuppression | 38 (15.5%) | |||
| Cavitated, necrotic or cystic | 119 (48.5%) | |||
| Necrosis at ROSE | 63 (25.7%) | |||
| >10 punctions | 109 (44.4%) | |||
| Chronic bronchial colonization | 5 (2%) | |||
| Immunosuppression (IS) (n; %) | 38 | |||
| Treatment immunosuppression | 24 (63.15%) | |||
| Chemotherapy | 12 (31.58%) | |||
| Long-term corticosteroids | 5 (13.15%) | |||
| Immunosuppressive treatment | 7 (18.42%) | |||
| Hereditary or acquired IS | 14 (36.85%) | |||
| HIV | 4 (10.5%) | |||
| Multi-complicated diabetes | 4 (10.5%) | |||
| Splenectomy | 2 (5.2%) | |||
| Agammaglobulinemia | 2 (5.2%) | |||
| Severe malnutrition | 2 (5.2%) | |||
| Number of Inclusion criteria (n; %) | ||||
| 1 | 168 (68.6%) | |||
| 2 | 66 (26.9%) | |||
| 3 | 11 (4.5%) | |||
| EBUS punctures (mean; SD) | 7.31 (SD 4.3) | 6.42 (SD 2.01) | 7.75 (SD 5.04) | P=0.048 |
| Node stations punctured (mean; SD) | 2.95 (SD 1.72) | 2.44 (SD 1.34) | 3.20 (SD 1.84) | P=0.01 |
Of the 245 risk factors patients, 168 (68.5%) had a single suspected risk factor, 66 (26.9%) had 2 different suspected risk factors and 1 (4.6%) met 3 different suspected risk factors. Of the 38 patients with immunosuppression, 12 (31.58%) were receiving chemotherapy, 5 (13.15%) long-term corticosteroids, and 7 (18.42%) immunosuppressive treatment. Of the remaining, 4 patients (10.5%) were HIV-1 positive under treatment, 4 patients (10.5%) had multi-complicated Diabetes Mellitus, 2 patients (5.2%) were splenectomised, 2 (5.2%) had agammaglobulinemia and 2 (5.2%) severe malnutrition.
The most frequent suspected risk factor was 10 or more than 10 punctures during EBUS-TBNA, which occurred in 109 patients (44.45%). The mean number of punctures per patient was 7.31 (SD 4.3) and a mean of 2.95 nodal stations (SD 1.72).
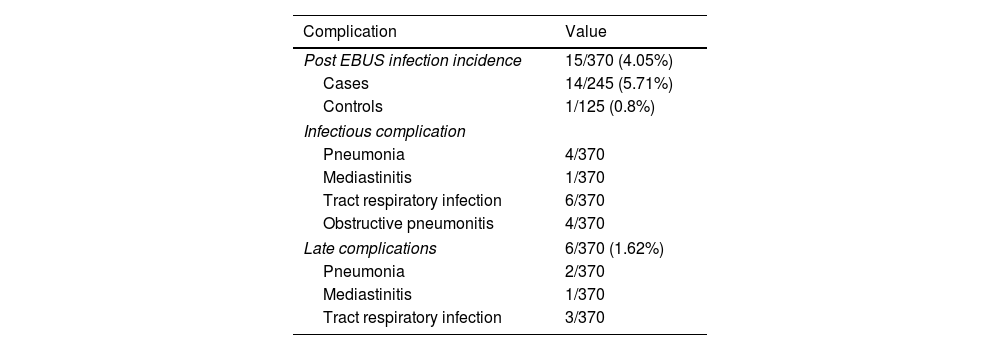
Infectious complicationsA total of 15 patients (4.05%) (Table 2), 14 were cases (5.71%) and 1 control (0.8%), presented a clinically relevant infectious complication: 4 pneumonias, 1 mediastinitis, 4 obstructive pneumonia and 5 respiratory tract infection, which resolved with antibiotic treatment, without any more severe complication. At one-month timepoint, 1 (0.27%) mediastinitis was detected within the first 15 days post-EBUS-TBNA as a late complication (which required hospitalization and surgical treatment, with subsequent good evolution) and 1 pneumonia (0.27%) 6 days after the test in a patient attended in the emergency department. Three (0.81%) respiratory tract infections and 2 (0.54%) pneumonias were detected by a telephone call at 30 days after EBUS-TBNA. Only one patient required hospitalization for severe complication and all cases resolved satisfactorily with antibiotics. No death occurred. Complications are described in Table 2.
Complications.
| Complication | Value |
|---|---|
| Post EBUS infection incidence | 15/370 (4.05%) |
| Cases | 14/245 (5.71%) |
| Controls | 1/125 (0.8%) |
| Infectious complication | |
| Pneumonia | 4/370 |
| Mediastinitis | 1/370 |
| Tract respiratory infection | 6/370 |
| Obstructive pneumonitis | 4/370 |
| Late complications | 6/370 (1.62%) |
| Pneumonia | 2/370 |
| Mediastinitis | 1/370 |
| Tract respiratory infection | 3/370 |
Nine patients (2.43%) presented fever immediately after EBUS-TBNA, self-limited in 6 patients and related to infectious complications in 3 patients (three cases of pneumonia). Fourteen (3.78%) patients presented fever during the 48h after EBUS, self-limited in 7 patients and related to infectious complications in another 7 (1 mediastinitis, 1 pneumonia, 2 obstructive pneumonias and 2 respiratory tract infection).
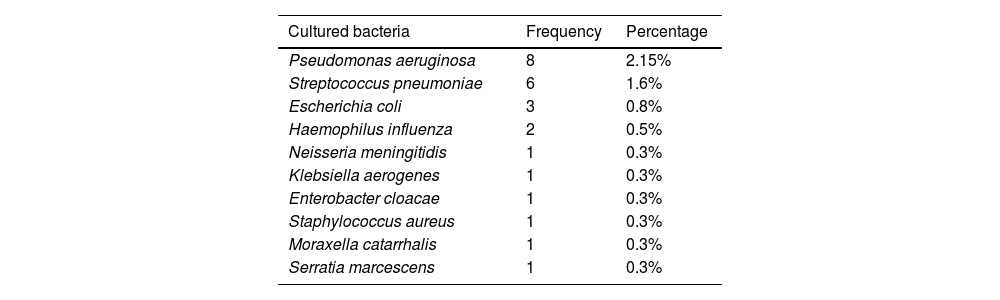
MicrobiologyOnly one patient (0.27%) had a positive blood culture for Streptococcus pneumonia, followed by a clinical diagnosis of pneumonia. Microbiological results are summarized in Table 3.
Microbiological profile.
| Cultured bacteria | Frequency | Percentage |
|---|---|---|
| Pseudomonas aeruginosa | 8 | 2.15% |
| Streptococcus pneumoniae | 6 | 1.6% |
| Escherichia coli | 3 | 0.8% |
| Haemophilus influenza | 2 | 0.5% |
| Neisseria meningitidis | 1 | 0.3% |
| Klebsiella aerogenes | 1 | 0.3% |
| Enterobacter cloacae | 1 | 0.3% |
| Staphylococcus aureus | 1 | 0.3% |
| Moraxella catarrhalis | 1 | 0.3% |
| Serratia marcescens | 1 | 0.3% |
Twenty-five (6.75%) BAS cultures with isolation of a germ were detected. Eight (2.15%) patients had Pseudomonas aeruginosa, 5 of them corresponded to patients with known chronic bronchial colonization, without clinical changes, and 3 were a new determination, and started nebulized treatment. Six patients (1.6%) had Streptococcus pneumoniae, two of them clinically followed by pneumonia, and four patients were asymptomatic and didn’t receive treatment. Four patients with a respiratory tract infection (1%) had 2 Escherichia coli and 1 Haemophilus influenzae, and received antibiotic treatment. One case of Staphylococcus aureus was considered as contamination and other single cases of Haemophilus influenzae, Escherichia coli, Neisseria meningitidis, Enterobacter cloacae, Klebsiellaaerogenes, Serratia marcescens and Moraxella catarrhalis isolation were asymptomatic and did not receive antibiotic.
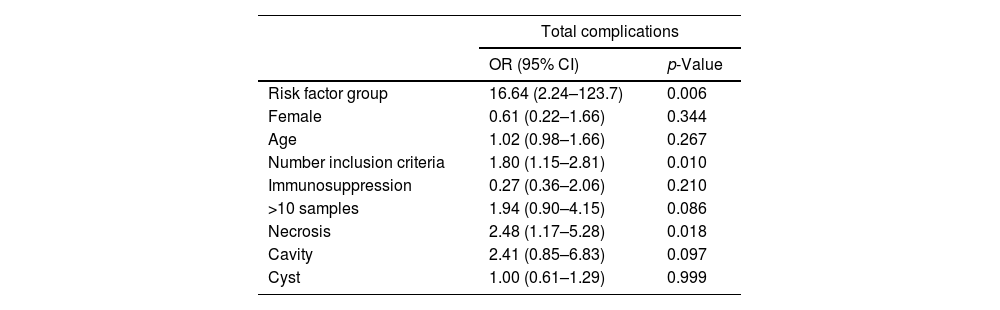
Potential risk factorsThrough logistic regression and multivariate analysis, all variables were analyzed (summarized values in Table 4). We appreciate an increased risk of complication in the risk factors group (OR 16.64, SD 17.03, p=0.006), and if there were more than one risk factor (p=0.010). We observed an increased risk of complications in patients with necrosis (OR 2.48, SD 0.95, p=0.018), even only in acute complications (OR 2.70, p=0.054). In patients with more than 10 punctures, a clear statistical tendency was detected, having an increased risk of complication (OR 1.94; p=0.086). We did not find any significant relationship of any other risk factor, neither with analytical alterations on the day of the test or at 3 days, nor with microbiological determinations, clinical symptoms changes such as fever or acute or late complications. (See supplementary data).
Association between risk factors and complications.
| Total complications | ||
|---|---|---|
| OR (95% CI) | p-Value | |
| Risk factor group | 16.64 (2.24–123.7) | 0.006 |
| Female | 0.61 (0.22–1.66) | 0.344 |
| Age | 1.02 (0.98–1.66) | 0.267 |
| Number inclusion criteria | 1.80 (1.15–2.81) | 0.010 |
| Immunosuppression | 0.27 (0.36–2.06) | 0.210 |
| >10 samples | 1.94 (0.90–4.15) | 0.086 |
| Necrosis | 2.48 (1.17–5.28) | 0.018 |
| Cavity | 2.41 (0.85–6.83) | 0.097 |
| Cyst | 1.00 (0.61–1.29) | 0.999 |
OR=odds ratio. ROSE=rapid on-site evaluation.
Our study demonstrates that infectious complications are low in EBUS-TBNA, even in a subgroup of patients at risk. To the best of our knowledge, this is the first prospective multi-center study to analyze the incidence of infectious complications and try to clarify the potential risk factors of post-EBUS infection.
The incidence of infectious complications described till the date in the general population ranges from 0.2% to 0.5%.8,14,18 Our study showed an incidence rate on infectious complications of 4.05% globally, and 5.71% in a selected group of patients, higher than previously described, which is probably due to the patient profile selected and the follow-up. In fact, the incidence is similar to the incidence of fever and infection after flexible bronchoscopy28 and the incidence of infection after EBUS-GS-TBB.24 Probably, the low number of minor complications in most studies is not reflecting current practice due to the lack of an accurate follow-up,22 and our study carries out a strict follow-up of the patients, being able to overestimate the incidence or showing the real incidence. In fact, Steinfort et al.13 described a bacteremia rate of 7% following EBUS-TBNA. In our study, maybe the 6 respiratory tract infection could be had not been diagnosed without monitoring or could be treated on an outpatient basis, and with the long-term complications.
Several publications on mediastinoscopy described minor in 4.7% to 5.4% patients29,30 and there is a significantly higher occurrence of major complications with mediastinoscopy in comparison with EBUS-TBNA,31 but no study refers to infectious complications.
The incidence of complications may vary depending on several factors28,30,31: follow-up of patients to detect possible complications is not often a routine procedure; existence of lack of documentation, especially for minor complications; the experience of the work group; and the aseptic handling of the material. Our study was prospective, with a group of control patients, all the data collected, it has been made a 3-day control and month follow-up, all bronchoscopists had a great experience, the needle and the stylet were handled aseptically in every puncture, and all patients were laryngeal mask and never suctioning above vocal cords, to avoid cross-contamination of floral bacteria of the oral cavity and pharynx.
Some previous studies in EBUS-TBNA suggests that chest CT showed centrally necrotic nodes could be a risk factor for mediastinitis after EBUS-TBNA,6,22,26 and some authors suggest avoiding puncture necrotic or cystic areas7 but the risk factors are not clear. Previous publications in the field of endoscopic ultrasonography (EUS) also recommend avoiding puncture of cystic-type lesions as well as the necrotic regions of tumors or nodes suggesting that they are poorly irrigated, so immunity is lowered, and the possibility of bacterial growth is greater.19–21 Our study also showed an increased risk of complication in patients with necrosis, so it could be important to avoid puncturing the necrotic areas during the procedure. There were no association in the other risk factors but we appreciate a statistically significant increased risk of complication in the risk factors group and if there were more than one risk factor, so perhaps a larger sample size is needed to demonstrate other risk factors, such as performing more than 10 punctures (a clear statistical tendency was found in our study).
Unlike all previous studies, where death or life-threatening consequences were observed,12,16,22 all the patients in our study had a favorable evolution. Expertise and strict monitoring on our part, with follow-up on the day of the test, at 3 days and at month, has probably favored this good control. In addition, no other complications such as pneumothorax, sustained hypoxia, airway injury or hemorrhage, happened. Determining the risk factors could be important to carry out strict monitoring. So, in patients with a risk factor, perhaps it would be pertinent a follow up.
According to the British Thoracic Society (BTS) guidelines, antibiotic prophylaxis is not recommended before bronchoscopy,28 and recent randomized controlled trials suggest that antibiotic prophylaxis is not recommended for EBUS-TBNA.32 In addition, recent study in EBUS-GS-TBB also showed that post-EBUS infection may occur despite the use of prophylactic antibiotics.25 Also, a newly reported meta-analysis in EUS-FNA concluded that prophylactic antibiotics do not seem to substantially reduce the risk of infections.33-35 In the present study, prophylactic antibiotics were not administered. Further studies are necessary to evaluate the need for prophylactic antibiotic administration, maybe in selected patients at risk.
Although the incidence of infectious complications with EBUS-TBNA in our study is higher than previously described (4.05% and 5.71%), it remains low, so the power of the study to identify particular risk factors is limited and this may be a limitation of the study. However, that it is a multicenter, prospective study with a control group, and this is a strong point of the study.
In conclusion, this study represents the first multicenter prospective study to evaluate EBUS-TBNA infectious complications and risk factors associated, choosing a subgroup of susceptible patients to try to clarify the potential risk factors of post-EBUS infection. Necrosis appears as the clearest potential risk factor and performing more than 10 punctures had a clear tendency. The incidence in the risk group was higher than usually, so they may require a strict monitoring, but globally remains low and no serious complication occurred, so reinforces the idea that EBUS-TBNA is a safe technique for assessment of the mediastinum. Future studies should expand this data and explore other risk factors.
FundingSupported by Sociedad Española de Neumología y Cirugía Torácica (SEPAR), (Scholarship PII NEUMOLOGIA INTERVENCIONISTA 005/2015 NI).
Conflict of interestsNone of the authors has any conflict of interest.
The authors thank all the research centers and their staff for their hard work in making this research possible.