This article analyzes the concept of inactive fibrotic lesions of presumed tuberculous origin (old healed tuberculosis), defined by radiological characteristics and a positive tuberculin skin test (TST), and we examine the evidence-based foundation for the indication of treatment of latent tuberculosis infection in these cases. We explore the risk of reactivation in older and recent literature, and the problems raised by the differential diagnosis with active tuberculosis with negative bacteriology. We also analyze data on the prevalence of fibrotic lesions in the recent literature. We examine the possible role of Interferon Gamma Release Assays (IGRAs) versus TST and other molecular antigen detection techniques in sputum that can aid in establishing the diagnosis and we discuss the current indications for chemoprophylaxis and the different options available. We propose diagnostic guidelines and therapeutic algorithms based on risk stratification by age and other factors in the management of radiological lesions that raise a differential diagnosis between fibrotic lesions and active pulmonary tuberculosis with negative bacteriology.
El presente artículo analiza el concepto de lesiones fibróticas inactivas de presumible origen tuberculoso (old healed tuberculosis), su definición por sus características radiológicas y la presencia de prueba de la tuberculina (TST) positiva. Se revisa el fundamento basado en la evidencia de la indicación de tratamiento de infección tuberculosa latente en estos casos, el riesgo de reactivación en la literatura antigua y reciente, así como los problemas que plantea el diagnóstico diferencial con la tuberculosis activa con bacteriología negativa. Se consideran los datos sobre prevalencia de lesiones fibróticas en la literatura reciente. Se analiza el posible papel de las técnicas de Interferon Gamma Release Assay (IGRA) versus TST, así como otras técnicas moleculares de detección antigénica en esputo que pueden ayudar a hacer el diagnóstico. Se analizan las actuales indicaciones de quimioprofilaxis, las diferentes opciones y se proponen algoritmos diagnósticos y terapéuticos basados en la estratificación del riesgo según la edad y otros factores, para manejar las lesiones radiológicas que plantean diagnóstico diferencial entre lesión fibrótica inactiva y tuberculosis pulmonar con bacteriología negativa.
Fibrotic lesions or scars, also known as old healed tuberculosis, are a common finding on chest X-rays and are universally accepted as an indication for treatment of latent tuberculous infection (TLTI).1,2 However, little information is available in the recent literature on the statistics of TLTI in fibrotic lesions, and references generally cite the major studies performed in the 1970s3,4 and publications from the International Union against Tuberculosis (IUAT)5 and Styblo et al.,6 dating from the 1980s.
Fibrotic scars are defined as lesions on chest X-ray larger than 5mm suggestive of old untreated pulmonary tuberculosis (PT) in patients without a previous diagnosis of PT. They are generally described as “well-defined” or “radiologically dense”, and consist of nodules, fibrosis-like linear images with or without retraction and bronchiectasis in the upper lobes and with no evidence of alveolar component and/or cavitations. Calcified primary complex, localized pleural thickening and/or isolated lateral costophrenic angle blunting have also been described, but these may be considered less significant and are excluded from some definitions.7,8
Most authors require a transverse induration on tuberculin skin testing (TST) of at least 5mm for the diagnosis of fibrotic lesions,1,5 although neither TST nor Interferon Gamma Release Assay (IGRA) is 100% sensitive.9 In our setting, up to 23% of the cases with negative TST have had PT confirmed with positive smear or culture (13% in the population with no risk factors, 60% in the HIV-infected population).10
Stable radiological findings over a period of 1 year were required as a criterion for diagnosis by the IUAT in 1982,5 although the American Thoracic Society (ATS) declared in 2003 that 3 months’ stability on X-ray was sufficient if sputum cultures were negative and there were no clinical symptoms.11
Images suggestive of PT on X-ray, whether known and/or treated, are not considered fibrotic lesions but rather “post-PT sequelae”, although at times no distinction is made between the two concepts,6,12 which can lead to some confusion.
Fibrotic lesions are important for 3 reasons:
- (1)
There is a risk of reactivation PT in the future.
- (2)
Misdiagnosis of fibrotic lesions can mask smear-negative active PT, and there is a risk of starting single-agent TLTI that may lead to acquired resistance or failure to treat. Conversely, misdiagnosis of fibrotic lesions as active PT may lead to the administration of unnecessary and potentially toxic medications.
- (3)
Fibrotic lesions on X-rays are not always indicative of tuberculosis (TB) and may be confounded with other unrelated disease entities that may present with the same radiological patterns.
These 3 points are discussed below.
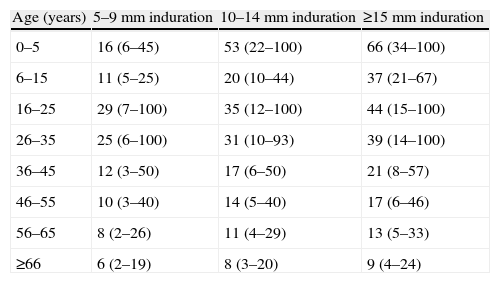
Risk of Reactivation of Fibrotic LesionsIn the IUAT study published in 1982 with 28000 participants over a 5-year follow-up, the annual risk of reactivation of TB was 0.286%, subdivided into 0.232% and 0.426% depending on whether lesions were smaller or larger than 2cm,2 respectively, on chest X-ray.5 In Rotterdam in 1984, Styblo et al.6 found an annual reactivation rate of 0.103% in 2895 fibrotic lesions monitored for 3 years. In USA reports from the 1970s, annual reactivation rates as high as 0.9% were recorded in a 5-year follow-up of 1992 cases.4 In 2004, Horsburgh13 published a metaanalysis of cohort studies performed in the USA between 1949 and 2003, stratifying the reactivation risk of subjects with positive TST. In old healed tuberculosis, he found a lifetime risk ranging from 66% in patients under 5 years of age with TST of 15mm or over, to 6% in patients over the age of 65 with TST between 5 and 9mm. The same author subsequently analyzed the reactivation rates of latent tuberculosis infection (LTI) in the USA, based on cases of PT confirmed by smear or culture not included in molecular clustering studies (cases attributed to endogenous reactivation and not recent transmission). He concluded that reactivation rates had fallen due to the almost total disappearance of fibrotic lesions in the USA-born population.14
No direct data on the current prevalence of fibrotic lesions in Spain are available. The results of widespread X-ray campaigns for the eradication of TB carried out between 1965 and 1973, revealing a prevalence of 5% in the general population,7 are no longer applicable, given the obvious improvement in the epidemiological situation since that time. However, it seems that prevalence may be as high as 13.8% among groups of poverty-stricken individuals and others that rely on social services.15 There are no systematic worldwide data on the country-specific prevalence of fibrotic lesions in the general population; available data are partial and derived from population reviews. For example, the radiological screening of 13379 Ethiopian immigrants in Israel found 257 (1.9%) patients with fibrotic lesions, of whom 15 (5.8%) developed active PT within one year.16 In an Indian study of 726 healthcare workers, 334 TST- and/or IGRA-positive subjects underwent radiological screening; 169 (23.2%) had calcified nodules and 37 (5%) had other lesions consistent with fibrotic scarring.12 These high rates are likely to be associated with the epidemiological situation in those countries, since the available partial data suggest that fibrotic lesions are more prevalent in areas with a high endemic presence of TB.
The risk of reactivation of fibrotic lesions depends on a series of factors, these being:
- (1)
The maturity of the lesions: according to the IUAT study,5 the risk in the untreated placebo group fell progressively over 5 years of follow-up compared to the first year.
- (2)
The lifetime risk of reactivation of fibrotic lesions diminishes significantly with age.13 Lesions are more likely to be old in the elderly, and given their reduced life expectancy there is less likelihood of reactivation. Conversely, in young subjects, lesions are more probably recent and this, along with longer life expectancy, leads to a higher risk of reactivation. In children, the concept of fibrotic scarring is complicated, since lesions can be presumed not to be old, and with the exception of some very specific cases, they should be diagnosed as active PT and treated accordingly.
- (3)
TST induration diameter is correlated with a greater risk of reactivation, particularly if it is larger than 15mm.13
- (4)
If conversion is recent and there is no old radiological evidence, the chances of the images corresponding to active PT are higher; however, conversion is an independent risk factor in itself.13,17,18
- (5)
The more extensive the scarring, the greater the bacillary load of the initial tuberculosis. The surface of the lesions on X-ray and the risk of reactivation are also statistically correlated.5 Lesions with cavitation, non-calcified adenopathies and/or pleural effusion should not be assumed to be fibrotic, even if smear or culture results are negative, as there is a strong possibility that they indicate active tuberculosis.
- (6)
Recent contact is a predictor of increased risk for active PT, irrespective of the presence or absence of fibrotic lesions on chest X-ray.13,17,18
- (7)
HIV infection is one of the most important immunosuppressive factors for the development of PT.2 Others (transplantation, corticosteroid treatment, anticancer chemotherapy, diabetes, etc.) may also be highly significant from a clinical point of view. After HIV infection, anti-TNF treatment is the most significant risk factor for LTI reactivation and may be associated with forms of disseminated tuberculosis.19 Post-transplantation immunosuppression is a major clinical concern in view of the high morbidity and mortality of TB in graft recipients. According to some guidelines, untreated fibrotic lesions in a donor lung could be a contraindication for transplantation.20
Before TLTI can be indicated in symptomatic patients or risk factor carriers, active PT and/or other diseases must be ruled out by conventional X-ray and/or by chest computed tomography (CT), bacteriological evaluation of spontaneous or induced sputum, or fiberoptic bronchoscopy with bronchoalveolar lavage (BAL) and aspirate for the detection of Mycobacterium tuberculosis (MTB). Chest CT may reveal cavitations, non-calcified adenopathies, pleural effusion or other lesions not visible on conventional chest X-ray that would rule out the diagnosis of fibrotic lesions. Before reaching a diagnosis and prescribing TLTI, culture results from sputum or any other specimens must be available and radiological stability over a minimum of 3 months must be confirmed.
When active tuberculosis can reasonably be ruled out, the TLTI regimen recommended by the ATS is 9 months of isoniazid (9H) or 4 months of rifampicin (4R) either in combination with isoniazid or as a single agent.1 In the 1980s, the IUALTLD set a gold standard of 12 months of isoniazid (12H).5 Later, after re-analyzing the data from the original studies, the ATS reduced the length of therapy to 9 months.1,21 The 4-month regimen of isoniazid plus rifampicin (4HR) has shown non-inferiority in terms of efficacy compared to conventional 12H.22 The great advantage of this regimen, aside from improved compliance and similar tolerability, is that it reduces the chance of acquired resistance developing if PT happens to be active but undetected.
Interferon Gamma Release Assay and Other Techniques in Fibrotic LesionsThere is little information on the value of IGRAs in fibrotic lesions. However, they may be more useful than TSTs as, being less affected by age, BCG vaccination and immunosuppression, they are more specific and probably more sensitive.23 A negativity rate of 16% has recently been reported for TST combined with IGRA in 193 subjects with fibrotic lesions, with positivity rates of 54.6% and 77.7%, respectively, and low concordance (K=0.25). There is no standard recommendation for the use of IGRA or TST in fibrotic lesions, although extrapolation of results from the management of high-risk patients suggests that it may be advisable to perform both tests and consider LTI if either is positive.24 By this criterion, if the TST were positive, it would not be necessary to perform the IGRA, which would be reserved for the case of a negative TST.
It is agreed that IGRAs cannot be used for diagnosing active PT, but they can give an indication of the presence of LTI.23 Nevertheless, the Tuberculosis Network European Trialsgroup study25 reported that IGRAs in BAL were useful for the diagnosis of PT with negative sputum smears. BAL was performed in 228 of the 347 patients with chest X-ray suggestive of PT and negative acid-fast bacilli (AFB) in sputum (or missing specimen) for determination of M. tuberculosis-specific ELISpot (T-SPOT.TB test) and amplification of M. tuberculosis-specific nucleic acids (NAAT) in BAL, in addition to conventional MTB cultures. In total, 71 cases of the active PT were found (56.3% with positive MTB culture) with a positive predictive value (PPV) for ELISpot of 87.2% and a negative predictive value (NPV) of 79.9% in confirmed PTs. For PTs diagnosed by both culture and clinical and radiological criteria, PPV was 90% and NPV was 83%. Moreover, the ratio of ESAT-6- and CFP-10-specific lymphocytes in BAL/blood on ELISpot was greater in active PT compared to inactive PT. The results were not affected by a previous history of PT. These findings are in line with other recent publications,26 although, as in the case of blood determinations,26 an IGRA study of BAL in HIV-carriers reported lower sensitivity and specificity due to the large proportion of indeterminate results.27 NAAT in sputum has been used as a tool for early diagnosis in both AFB smear-positive and negative PT, and has a sensitivity of 79.3% and specificity of 80.3% in smear-negative cases.28 The CDC reports a sensitivity of between 50% and 80%, depending on the authors, in smear-negative, culture-positive cases, and suggests that in settings where NAAT testing is routinely used, the results may affect the clinical decision in 20% to 50% of the cases.29 Furthermore, Xpert® MTB/RIF has been used for the early detection of active PT with initial negative smear, showing sensitivity of 75% and specificity of 99% in cases with positive cultures only.30 These techniques may be of assistance in the early differentiation of fibrotic lesions from active PT, but their role in clinical practice is not yet defined and positive MTB culture remains the gold standard.
Diagnostic and Therapeutic OrientationThe differential diagnosis of fibrotic lesions includes all pulmonary processes that may present with radiological features similar to PT (non-tuberculous mycobacteria, histoplasmosis, nocardiosis, coccidiodomycosis, post-pneumonia fibrosis, upper lobe bronchiectasis, interstitial diseases, sarcoidosis, diverse granulomatous diseases), and of course, active PT. Positive TST and/or IGRA supports the tuberculous etiology, but cannot be relied on for diagnosis. Careful clinical evaluation and appropriate complementary examinations must be performed to rule out other diseases, taking into consideration the relative risk of tuberculosis (belonging to a risk group, immigrants from countries with high endemic levels, contact with TB patients, immunosuppression), other lung diseases and typical features on X-ray.
When other diseases have been reasonably ruled out, there are several therapy options if clinical symptoms are suggestive of active tuberculosis.
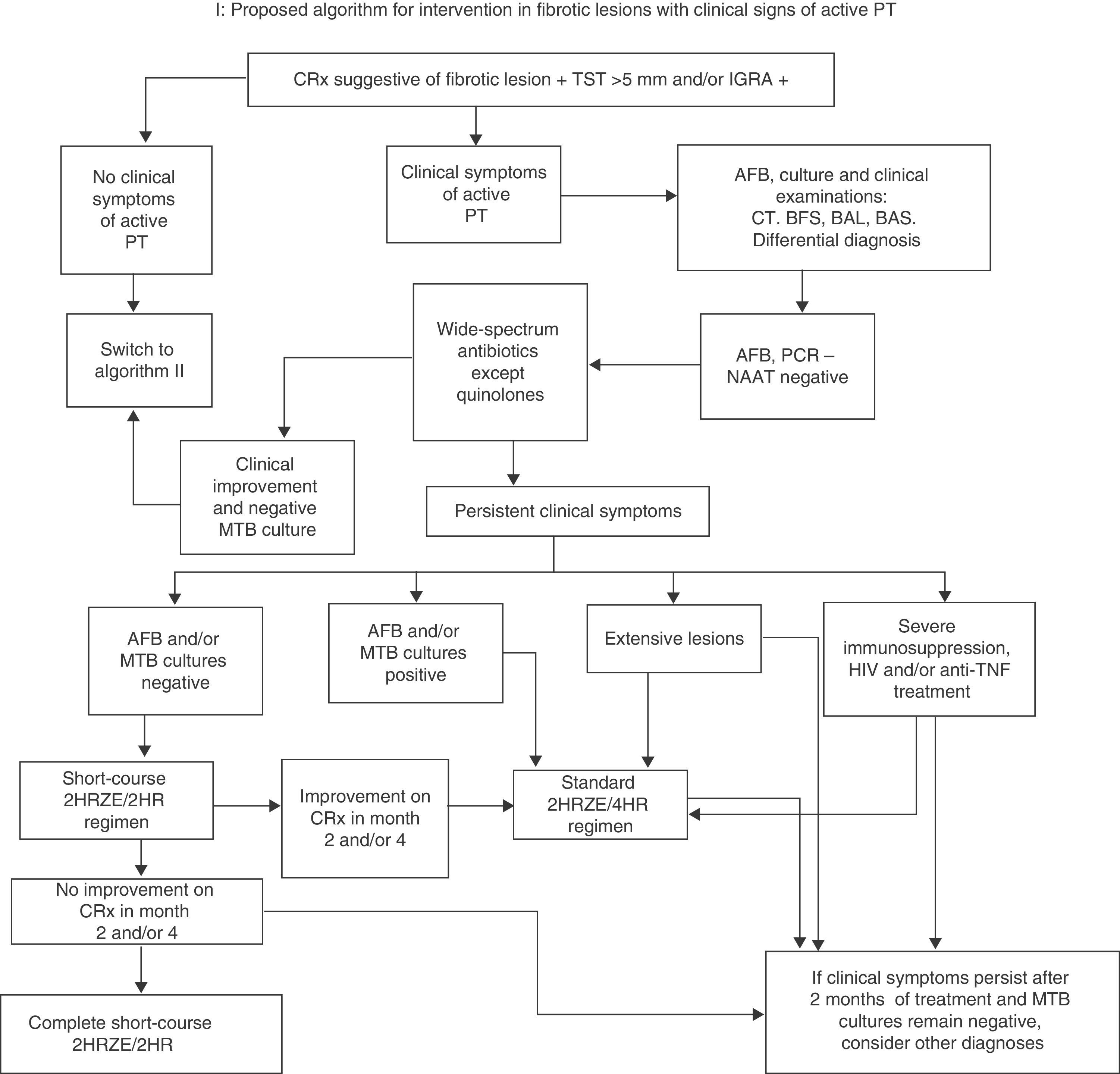
The first, if the initial AFB smear is positive, is to start conventional treatment (2HRZE/4HR). If the initial smear is negative, the World Health Organization (WHO) algorithms (2007 and 2009) advise the administration of wide-spectrum antibiotics (with the exception of quinolones, to prevent MTB resistance), monitoring progress until culture results are available.31,32 If the radiological picture is highly suggestive and other diagnoses are unlikely, but it is unclear whether the lesion is fibrotic or active TB, it is preferable to start standard treatment with 2HRZE and reassess after 2 months. If, at that time, sputum cultures are negative, radiology is stable and there are no risk factors, give H and R for a further 2 months (4 months in total). The lesion can be assumed to be fibrotic if it remains unchanged in the final X-ray performed at 4 months. The ATS accepts the short-course 4-month regimen for active PT with negative culture,11,33 even if there has been radiological improvement. In fact, a lack of improvement on X-ray at 2 months would require a reevaluation of the PT diagnosis. However, other programs suggest that if there has been radiological improvement in month 2 and/or 4, or if there are other additional factors (cavitated and/or extensive lesions, young age, immunosuppression, etc.), the full 6-month 2HRZE/4HR regimen should be completed and the case should be classified as culture-negative active PT.34 This approach seems more advisable, in view of the lack of controlled studies (Fig. 1).
Proposed algorithm for intervention in fibrotic lesions with clinical signs of active PT. BAL: bronchoalveolar lavage; BAS: bronchial aspirate; FBS: fiberoptic bronchoscopy; AFB: acid-fast bacilli smear; IGRA: Interferon Gamma Release Assay; MTB: Mycobacterium tuberculosis; NAAT: nucleic acid amplification test; PCR: polymerase chain reaction; CRx: chest X-ray; CT: chest tomography; PT: pulmonary tuberculosis; TST: tuberculin skin test.
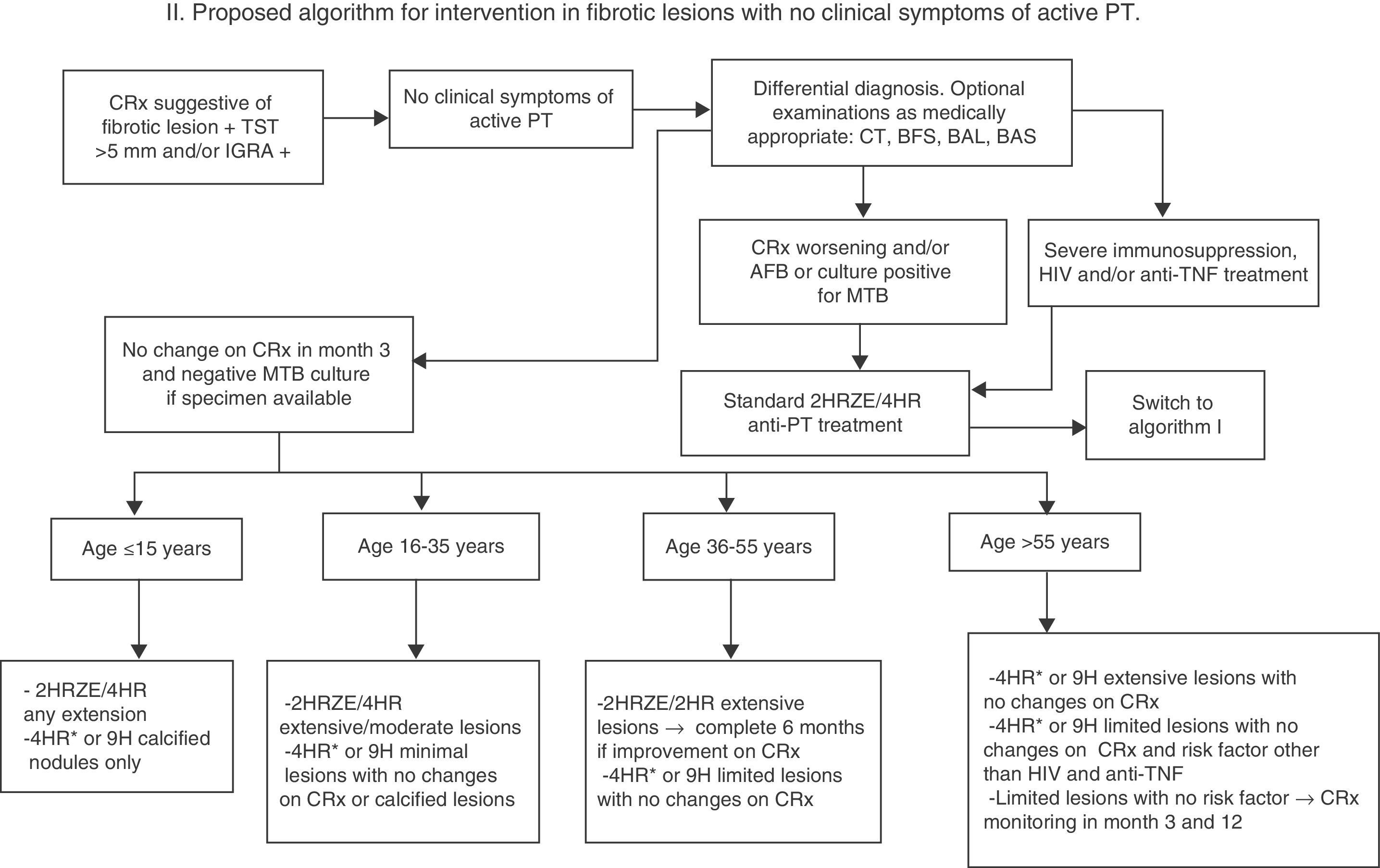
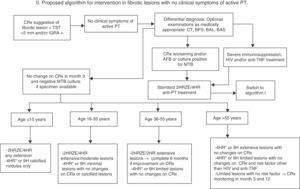
If there are no clinical symptoms to suggest active tuberculosis, a follow-up X-ray should be performed after 3 months (1 and 3 months if lesions are extensive) while waiting for culture results, if a specimen can be obtained (Fig. 2). If the chest X-ray shows no change, no images suggestive of other etiology are revealed and cultures are negative, or no specimen is available, a diagnosis of inactive fibrotic lesion can be safely made. There is no evidence based on controlled studies to suggest a different strategy in this case, and all the guidelines specify the administration of only conventional TLTI with no time limitations, but the risk obviously varies widely depending on associated factors (age, extension of lesions, immunosuppression, etc.). In any case, the risk of possible drug toxicity must be weighed against the benefit of preventing reactivation, and administration of TLTI in possibly active TB must always be avoided as far as possible. For this reason, we propose in this paper a regimen based on the stratification of the lifetime risk of reactivation of fibrotic lesions by age, grouped according to Horbsburg13 (Table 1), and other risk factors.
- (A)
For the >55 age group, with their higher risk of liver toxicity and lower risk of reactivation, TLTI with 4HR or 9H for extensive lesions with no suspected activity can be recommended. Also consider TLTI with 4HR or 9H for limited lesions if there are risk factors other than HIV, anti-TNF treatment or transplantation. In the absence of risk factors, if lesions are limited, clinical and radiological monitoring at 3 and 12 months may be indicated.
- (B)
For the 36–55 age group, TLTI with 4HR or 9H may be recommended if lesions are limited and there is no suspected activity. If lesions are extensive, the short-course 2HRZE/2HR regimen may be indicated, and if there is radiological improvement after 2 and 4 months, the regimen should be extended to 6 months.
- (C)
For the under-36 age group, the wisest approach for all practical purposes is to consider fibrotic lesions as recently acquired PT, and to start a standard 2HRZE/4HR regimen. Exceptionally, if lesions are minimal or calcified nodules with no changes on X-ray, TLTI with 4HR or 9H may be initiated.
- (D)
For children under 15, it is very unclear whether the concept of inactive fibrotic lesion is ever acceptable, even if lesions are minimal, and the standard 2HRZE/4HR regimen should be prescribed, reserving TLTI with 4HR or 9H for calcified nodules only.
Proposed algorithm for intervention in fibrotic lesions with no clinical symptoms of active PT. 4HR*: regimen of choice, given lower risk of induced resistance if the patient was incorrectly classified and has culture-negative, active TB. BAL: bronchoalveolar lavage; BAS: bronchial aspirate; BFS: fiberoptic bronchoscopy; AFB: acid-fast bacilli smear; IGRA: Interferon Gamma Release Assay; MTB: Mycobacterium tuberculosis; NAAT: nucleic acid amplification test; PCR: polymerase chain reaction; CRx: chest X-ray; CT: chest tomography; PT: pulmonary tuberculosis; TST: tuberculin skin test.
Percentage Lifetime Risk of Developing Active PT in Patients With Fibrotic Lesions (95% Confidence Interval) According to Tuberculin Skin Test Induration Diameter.
| Age (years) | 5–9mm induration | 10–14mm induration | ≥15mm induration |
| 0–5 | 16 (6–45) | 53 (22–100) | 66 (34–100) |
| 6–15 | 11 (5–25) | 20 (10–44) | 37 (21–67) |
| 16–25 | 29 (7–100) | 35 (12–100) | 44 (15–100) |
| 26–35 | 25 (6–100) | 31 (10–93) | 39 (14–100) |
| 36–45 | 12 (3–50) | 17 (6–50) | 21 (8–57) |
| 46–55 | 10 (3–40) | 14 (5–40) | 17 (6–46) |
| 56–65 | 8 (2–26) | 11 (4–29) | 13 (5–33) |
| ≥66 | 6 (2–19) | 8 (3–20) | 9 (4–24) |
For HIV-positive patients, patients of any age receiving anti-TNF biologics, and immunosuppressed subjects (transplant recipients on immunosuppressants, anticancer chemotherapy, etc.), it is inadvisable to accept a diagnosis of fibrotic lesion. Administration of the standard 2HRZE/4HR regimen is preferable, given the high risk of reactivation. Under some tuberculosis programs, the reason for not starting the standard regimen when X-ray is suggestive of PT must be reported,34 and any prescription of chemoprophylaxis, especially in single-agent regimens (9H, 12H, 4R), must be justified. If bacterial resistance is suspected (recent contact with a resistant patient or other circumstances), the recommendation is to start full treatment tailored to the suspected resistance profile in high-risk patients (children, immunosuppressed subject, etc.) and to withhold treatment and initiate clinical and radiological monitoring in all others.
ConclusionIn our setting, the standard approach to fibrotic lesions is to either treat as active PT and prescribe standard 2HRZE/4HR, or else to withhold treatment (with at most clinical and radiological monitoring). In some very rare cases, TLTI is prescribed based on studies published 30 years ago or more –practically nothing new has been published in the area since 1982. It is patently obvious that, considering the risk factors for TB reactivation and possible confusion with active TB, consensus on treatment options must be reached, and published studies must be adapted to current clinical practice.
Conflicts of InterestThe authors state that they have no conflict of interest directly or indirectly related with the contents of this manuscript.
Please cite this article as: Solsona Peiró J, de Souza Galvão ML, Altet Gómez MN. Lesiones fibróticas inactivas versus tuberculosis pulmonar con bacteriología negativa. Arch Bronconeumol. 2014;50:484–489.