Frequent-exacerbator COPD (fe-COPD) associated with frequent hospital admissions have high morbidity, mortality and use of health resources. These patients should be managed in personalized integrated care models (ICM). Accordingly, we aimed to evaluate the long-term effectiveness of a fe-COPD ICM on emergency room (ER) visits, hospital admissions, days of hospitalization, mortality and improvement of health status.
MethodsProspective-controlled study with analysis of a cohort of fe-COPD patients assigned to ICM and followed-up for maximally 7 years that were compared to a parallel cohort who received standard care. All patients had a confirmed diagnosis of COPD with a history of ≥2 hospital admissions due to exacerbations in the year before enrollment. The change in CAT score and mMRC dyspnea scale, hospital admissions, ER visits, days of hospitalization, and mortality were analyzed.
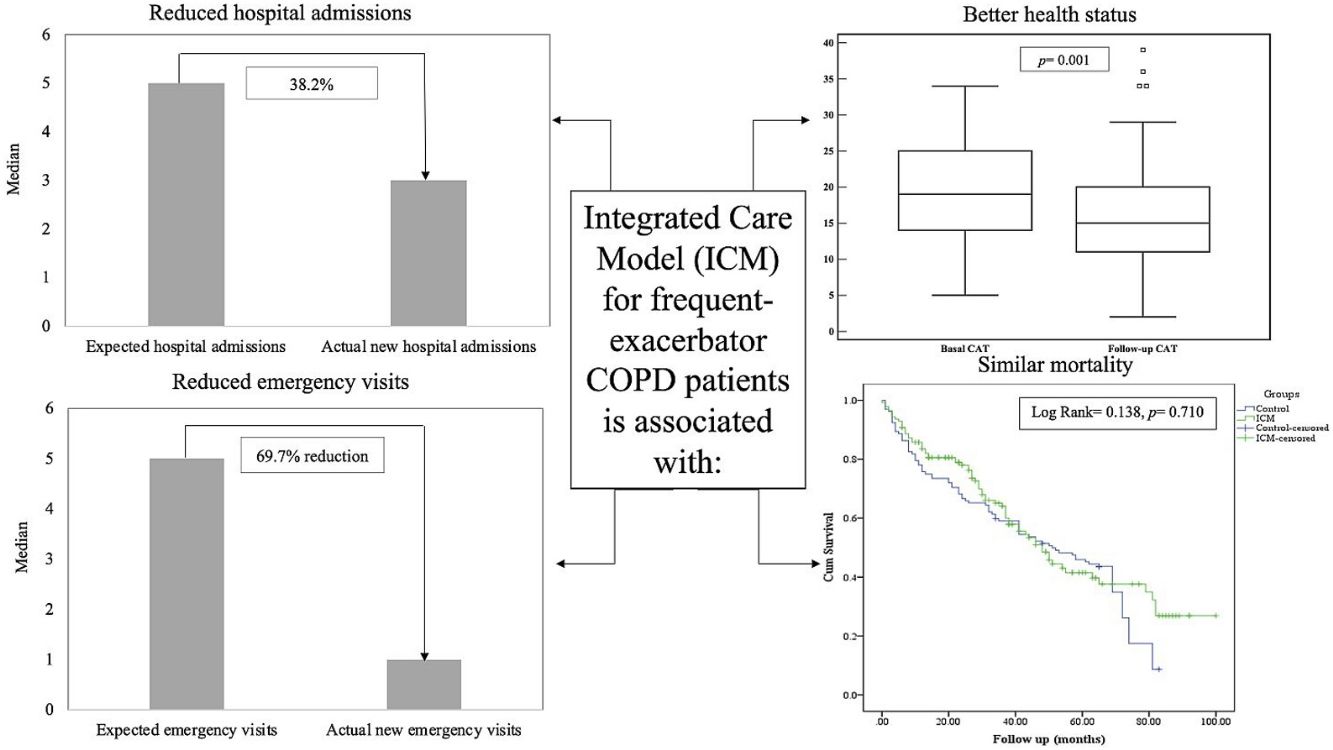
Results141 patients included in the ICM were compared to 132 patients who received standard care. The ICM reduced hospitalizations by 38.2% and ER visits by 69.7%, with reduction of hospitalizations for COPD exacerbation, ER visits and days of hospitalization (p<0.05) compared to standard care. Further, health status improved among the ICM group after 1 year of follow-up (p=0.001), effect sustained over 3 years. However, mortality was not different between groups (p=0.117). Last follow-up CAT score>17 was the strongest independent risk factor for mortality and hospitalization among ICM patients.
ConclusionsAn ICM for fe-COPD patients effectively decreases ER and hospital admissions and improves health status, but not mortality.
La EPOC con agudizaciones frecuentes (EPOC-AF), que se asocia a ingresos hospitalarios recurrentes, presenta altas tasas de morbilidad y mortalidad, y un importante uso de los recursos sanitarios. Estos pacientes deberían ser tratados en modelos de atención integral (MAI) personalizada. Por este motivo, nuestro objetivo fue evaluar la efectividad a largo plazo de un MAI para EPOC-AF valorando las visitas a urgencias, los ingresos hospitalarios, los días de hospitalización, la mortalidad y la mejora del estado de la salud.
MétodosEstudio prospectivo controlado que analizó una cohorte de pacientes con EPOC-AF incluidos en un MAI y en seguimiento durante un máximo de 7 años en comparación con una cohorte paralela que recibió atención estándar. Todos los pacientes tenían diagnóstico confirmado de EPOC y antecedentes de ≥2 ingresos hospitalarios por agudizaciones durante el año anterior a su inclusión en el estudio. Se analizaron los cambios en la puntuación del CAT© y en la escala de disnea del MRC, en los ingresos hospitalarios, las visitas a urgencias, los días de hospitalización y la mortalidad.
ResultadosSe compararon 141 pacientes incluidos en el MAI con 132 pacientes que recibieron atención estándar. El MAI redujo las hospitalizaciones en un 38,2% y las visitas a urgencias en un 69,7%, mostrando reducción de las hospitalizaciones por exacerbación de la EPOC, las visitas a urgencias y los días de hospitalización (p<0,05) en comparación con la atención estándar. Además, el estado de salud mejoró en los pacientes del grupo del MAI después de un año de seguimiento (p=0,001), un efecto que se mantuvo durante 3 años. Sin embargo, la mortalidad no fue diferente entre ambos grupos (p=0,117). Una puntuación en el CAT©>17 en el último control de seguimiento fue el factor independiente de riesgo más fuertemente asociado a la mortalidad y la hospitalización de los pacientes en el MAI.
ConclusionesUn MAI para pacientes con EPOC-AF reduce eficazmente los ingresos hospitalarios y en urgencias, y mejora el estado de salud, pero no la mortalidad.
Chronic obstructive pulmonary disease (COPD) is a chronic disease1 associated with significant morbidity and mortality, especially in those with frequent exacerbations.2 Acute exacerbations of COPD (AECOPD) result in significant loss of quality of life and lung function3 as well as high morbidity, mortality and use of hospital resources.4,5 Despite the advances in therapy and efforts to prevent exacerbations, the burden and prevalence of severe exacerbations remain high.6
The “frequent exacerbator” COPD patient is a well identified phenotype defined by consensus by the presence of 2 or more exacerbations or one hospital admission during the preceding year.1,7 Patients with more severe COPD had more frequent AECOPD.8 Moreover, history of previous exacerbation is an important predictor of subsequent exacerbations.8,9 Severe exacerbations leading to hospital admissions have an important impact on the quality of life and mortality of these patients.10–12
In the last decade, the importance of providing special care to the frequent-exacerbator COPD patient was emphasized. Various care models targeting these patients reported a reduction of health-care resources utilization in terms of emergency room (ER) visits and hospital admission as well as improvement of quality of life among this group of patients.13–16 The majority of these programs provided schedule visits and on-demand visits14–16; while other programs considered further telemonitoring through medical call centers.13,15 However, it has been claimed that future models of care should be personalized – providing patient education aiming at behavior changes, identifying and treating co-morbidities, and including outcomes that measure quality of care rather than focusing only on readmission quantity within 30 days.17
We hypothesized that a personalized and integrated care model (ICM) for frequent-exacerbator COPD patients (fe-COPD) could provide a sustained and better quality of care for this group of patients and reduce the overuse of healthcare resources. Accordingly, we aimed to: (a) evaluate longitudinally the impact of a long-term fe-COPD ICM on ER visits, hospital admissions, and days of hospitalization; (b) investigate the effects of the ICM on health status and all-causes of mortality; (c) evaluate the risk factors of mortality among the whole studied population and ICM group.
MethodsStudy design and subjectsProspective controlled study that recruited patients from outpatient's respiratory clinics after being discharged from the hospital due to AECOPD in a tertiary teaching hospital between 2012 and 2019. Patients were identified after two or more hospital admissions per year due to AECOPD and were offered to join a fe-COPD ICM if they fulfilled the following inclusion criteria: (1) patients with diagnosis of COPD according to GOLD criteria,1 able to attend scheduled visits to hospital, (2) able to contact the ICM team by phone and, (3) able to follow the treatment instructions. Patients were excluded if they (1) had non-respiratory comorbidity that substantially affected the prognosis of the disease, (2) lived out of the area of influence of the hospital, (3) lived in a nursing home or jail, (4) suffered from deteriorative cognitive function or psychiatric illness that affected their mental capacity, or (5) had socio-familial conditions that affected the access to the hospital or to attend the scheduled visits. The control group were similarly COPD patients diagnosed according to GOLD criteria with history of ≥2 AECOPD required hospitalization and fulfilled inclusion criteria with no exclusion criteria for ICM but they were unwilling to be included in the ICM, or refused to contact the program team through the phone and preferred to come directly to ER, or preferred to continue with their primary physician of care. The control group underwent standard care in the same hospital with scheduled outpatient visits in Primary and Specialized Care and were included in a database as controls and analyzed retrospectively. Exacerbations were defined, and all patients received treatment, according to GOLD recommendations.1 The study was approved by the research board of the participating hospital and all the participants signed a written informed consent.
Frequent-exacerbator COPD integrated care modelThe fe-COPD patients enrolled in the ICM were assigned to a nurse-led program under medical supervision that included personalized-structured visits with social, nutritional, rehabilitation, educational (including smoking cessation when required) and functional assessment. Also, patients were provided with a fast-track access to medical care in case of new respiratory symptoms by a direct telephone call with the ICM team. The evaluation and plans with every patient were shared with their Primary Care nurse through a web-based chronic disease management package included in the local health care electronic record system.
At first visit, patients were subjected to: (1) complete medical history with assessment of comorbidities and evaluation of health status with COPD assessment test (CAT), the modified Medical Research Council (mMRC) dyspnea scale, smoking history, and history of previous exacerbations; (2) forced spirometry, lung volumes and DLCO (if there was not a previous one within the 6 months prior to admission to the program), ADO index (age, dyspnea and airway obstruction), 6-minute walking test, arterial blood gases and BODE index was calculated; (3) sputum sample for microbiological evaluation; (4) revision of inhalation techniques and compliance by pharmacy refills in the electronic prescription records; (5) education about healthy habits, alert signs of exacerbations and aiming at behavior changes including smoking cessation; (6) nutritional status and need of dietary assessment; (7) physical activities and need of rehabilitation; and (8) assessment of psychosocial support and identification care-givers with coaching with patient and family support. All patients were followed-up on regular basis at scheduled visits every 3 months (or earlier if required) with evaluation of symptoms, vital signs, mMRC dyspnea scale, CAT score, O2 saturation and re-checking of inhalation techniques by specialized nurse at each single visit. Identification and treatment of new comorbidities, compliance of treatments, physical activity and psychosocial needs were also assessed in every scheduled visit. Moreover, patients included in the program were able to contact the ICM team from Monday-to-Friday through telephone calls for consultation about their symptoms outside the scheduled visits. Based on their new presenting symptoms, the team decided whether the patient needed to come to hospital for medical evaluation and accordingly further action was considered (online supplement fig. 1). Out-of-hours, patients requiring urgent medical attention had to go to the ER at their Primary Care center or hospital.
Variables and outcomesNew hospital admissions, domiciliary hospitalization, ER visits, courses of antibiotic and systemic corticosteroids as well as mortality were recorded for every patient during follow-up for further analysis. All variables were analyzed as cumulative variables throughout the whole follow-up duration not as annual frequency. Average days of hospitalization were recorded for each patient and analyzed. Patients with AECOPD who were managed by the ICM team as outpatient were considered as avoided ER visits. When patients fulfilled criteria for hospitalization, providing that they were not on acute respiratory failure, and were conscious with good home support, they were managed as outpatient instead with close monitoring by the program team. This was considered as avoided admissions. Baseline and follow-up values of mMRC and CAT were collected prospectively in the ICM group.
Statistical analysisThe data were expressed as median and interquartile range (IQR) or number and percentage (%) as appropriate unless otherwise stated. Mann–Whitney, unpaired t-test and chi-square tests were used in the comparison between both groups as appropriate. A percentage of reduction of new hospital admissions and ER visits was calculated to study the effect of the ICM as the relation between measured new hospital admission or ER visits/expected hospitalizations or emergency visits (i.e. actual new hospital admission or emergency visits+avoided admissions or ER visits).
C-statistic was used to identify the best cut-off point for CAT score as predictor of mortality among fe-COPD ICM patients. Logistic univariate and multivariate analysis were used to evaluate the risk factors for new hospital admissions and mortality among fe-COPD ICM patients. Odd ratio (OR) and confidence interval 95% (CI95%) were calculated. Cumulative mortality within 7 years of enrollment in the ICM was estimated using Kaplan–Meier survival analysis. A two-tailed p value<0.05 was considered statistically significant. SPSS package (Version 22.0. Armonk, NY: IBM Corp) was used for all analyses.
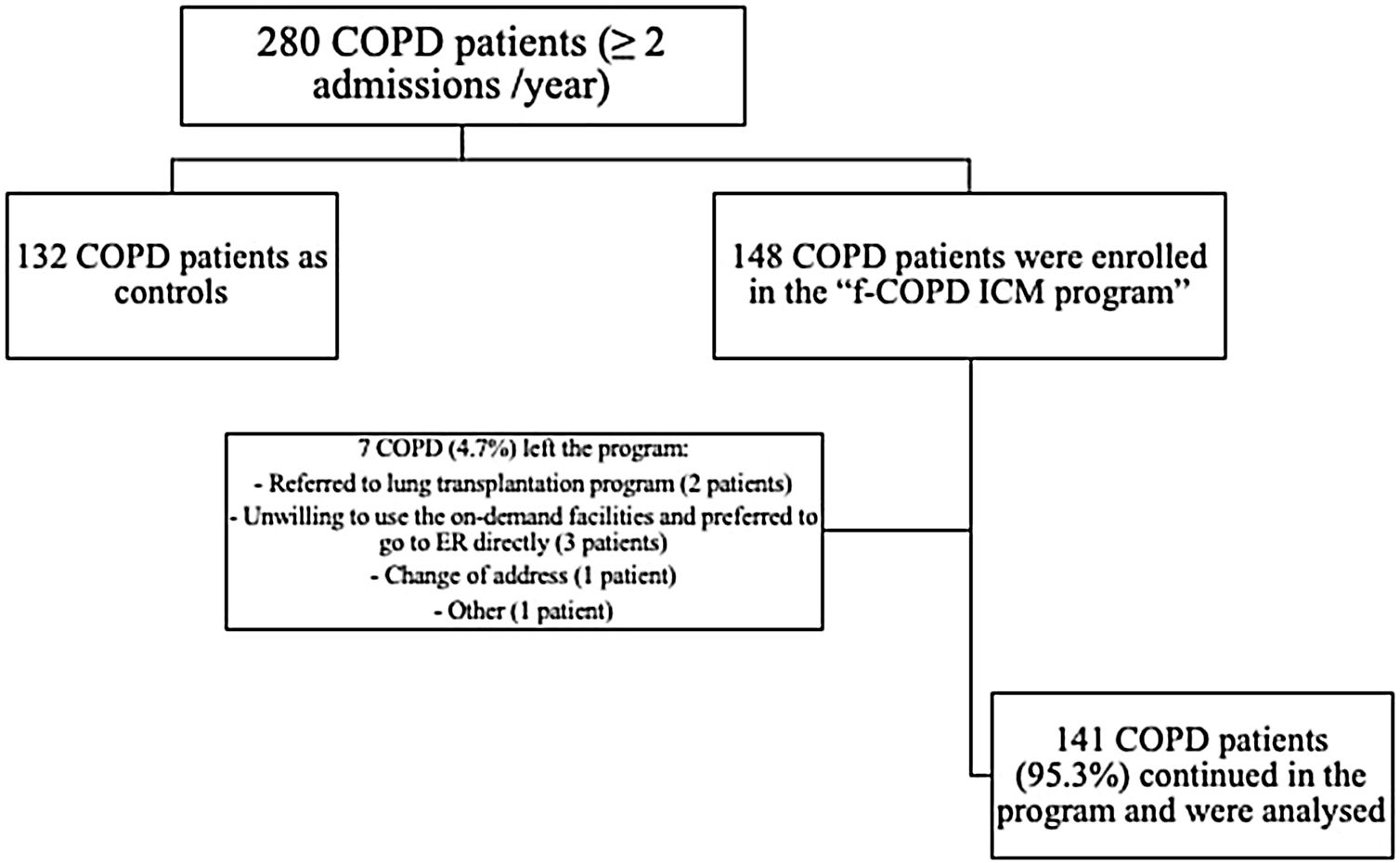
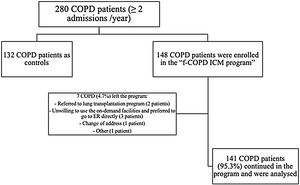
ResultsCharacterization of the population280 fe-COPD patients were followed-up for a median of 37 months (IQR=14–65 months) and maximally 7 years. 148 fe-COPD patients were assigned to fe-COPD ICM, and 132 fe-COPD were unwilling to join the program and considered as a control group receiving standard medical care. Seven fe-COPD from the ICM group (4.7%) abandoned the program due to the occurrence of exclusion criteria during follow-up; accordingly, 141 patients completed the program and were analyzed (Fig. 1).
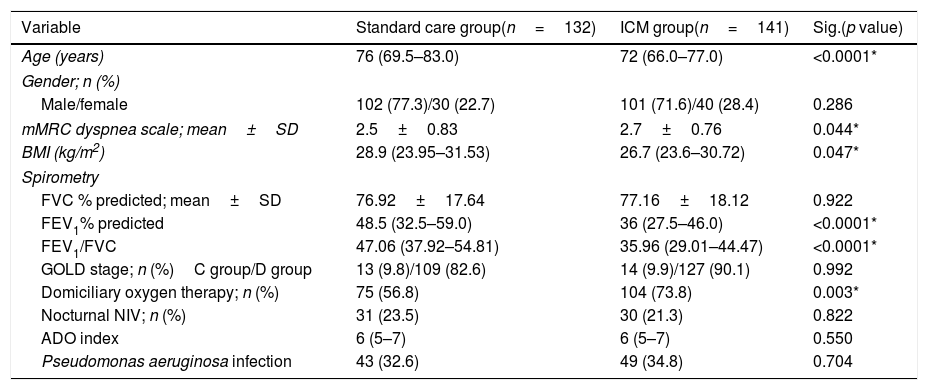
Table 1 shows the baseline clinical and functional characteristics of the studied population. All included fe-COPD patients were predominantly GOLD D in both groups with similar proportion of patients with airway colonization by Pseudomonas aeruginosa between both groups. Further, the ADO index was similar between both groups (p=0.550, Table 1). Noteworthy, patients in the fe-COPD ICM group were slightly younger (72 (IQR=66.0–77.0) vs 76 (IQR=69.5–83.0) years), with more severe airway obstruction and lower BMI than the control group (Table 1). Seventy-five patients (56.8%) of control group were on domiciliary oxygen therapy versus 104 patients (73.8%) of ICM group (p=0.003); while 23.5% versus 21.3% respectively used nocturnal non-invasive ventilation (p>0.05, Table 1).
Baseline characteristics of the studied population.
| Variable | Standard care group(n=132) | ICM group(n=141) | Sig.(p value) |
|---|---|---|---|
| Age (years) | 76 (69.5–83.0) | 72 (66.0–77.0) | <0.0001* |
| Gender; n (%) | |||
| Male/female | 102 (77.3)/30 (22.7) | 101 (71.6)/40 (28.4) | 0.286 |
| mMRC dyspnea scale; mean±SD | 2.5±0.83 | 2.7±0.76 | 0.044* |
| BMI (kg/m2) | 28.9 (23.95–31.53) | 26.7 (23.6–30.72) | 0.047* |
| Spirometry | |||
| FVC % predicted; mean±SD | 76.92±17.64 | 77.16±18.12 | 0.922 |
| FEV1% predicted | 48.5 (32.5–59.0) | 36 (27.5–46.0) | <0.0001* |
| FEV1/FVC | 47.06 (37.92–54.81) | 35.96 (29.01–44.47) | <0.0001* |
| GOLD stage; n (%)C group/D group | 13 (9.8)/109 (82.6) | 14 (9.9)/127 (90.1) | 0.992 |
| Domiciliary oxygen therapy; n (%) | 75 (56.8) | 104 (73.8) | 0.003* |
| Nocturnal NIV; n (%) | 31 (23.5) | 30 (21.3) | 0.822 |
| ADO index | 6 (5–7) | 6 (5–7) | 0.550 |
| Pseudomonas aeruginosa infection | 43 (32.6) | 49 (34.8) | 0.704 |
ICM: integrated care model, fe-COPD: frequent-exacerbator COPD, mMRC: modified medical research council, FEV1: forced expiratory volume in 1 second, FVC: forced vital capacity, L: liter, n: number, SD: standard deviation, ADO index: age, dyspnea, and airway obstruction index, NIV: non-invasive ventilation.
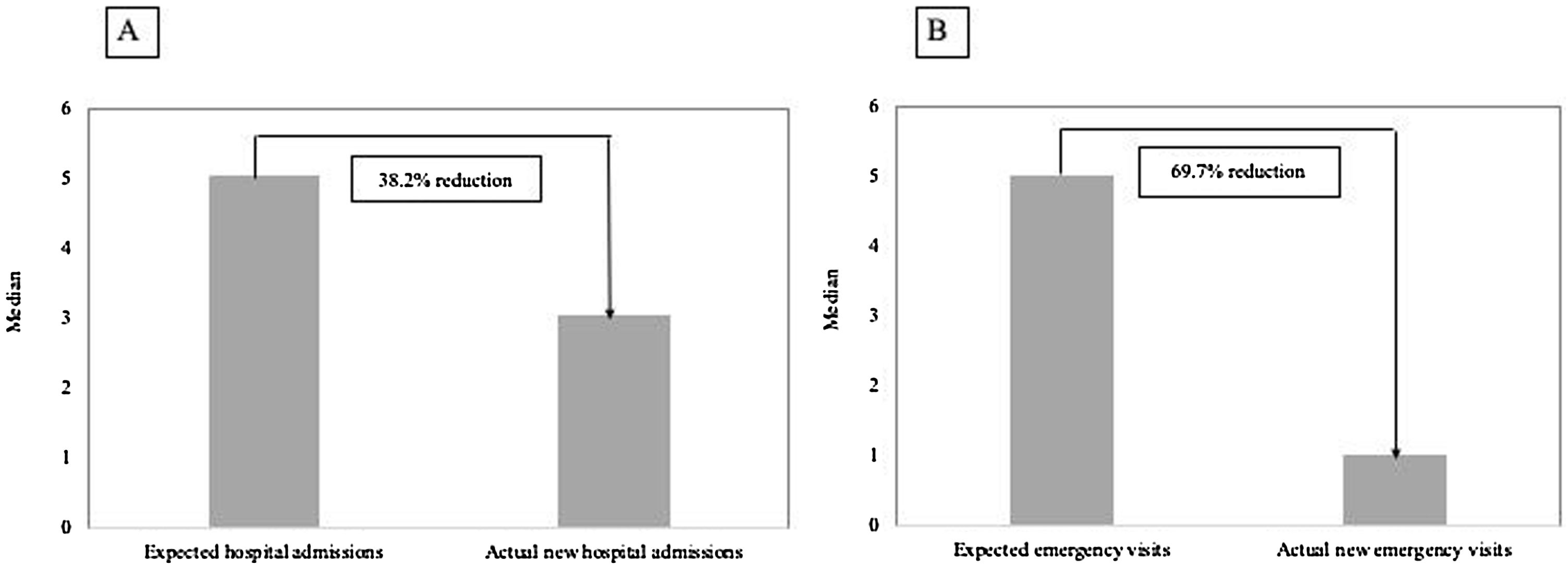
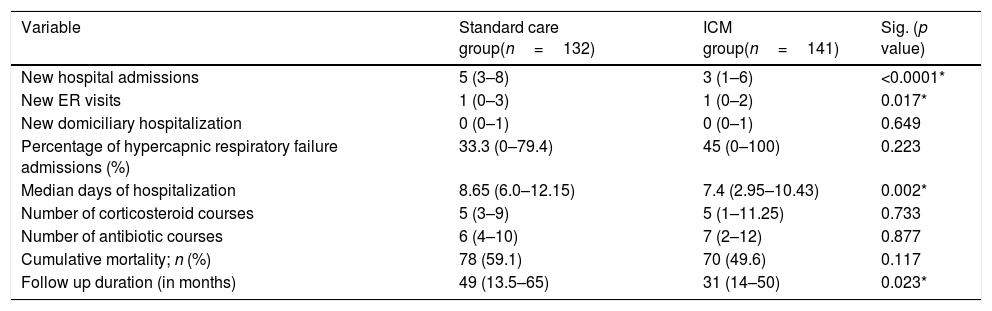
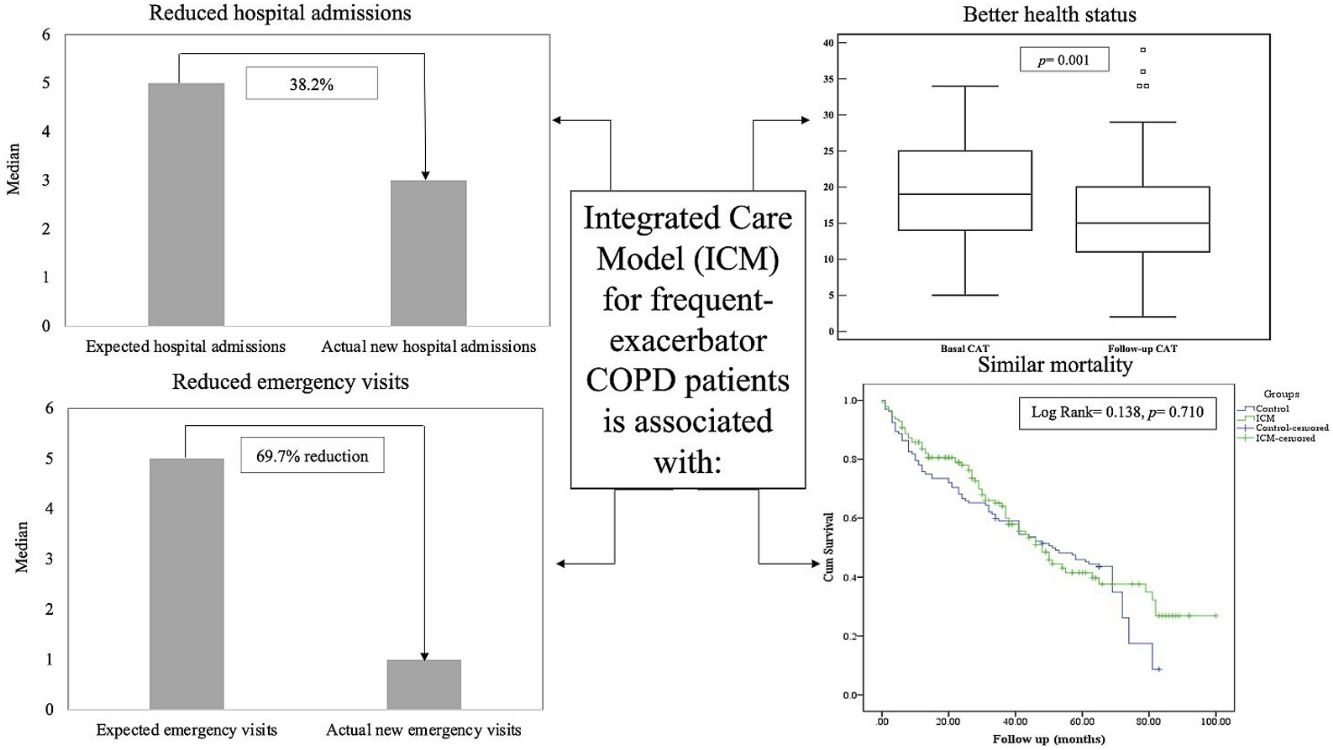
The median number of AECOPD was 6 (IQR=3–11) among the ICM group; however, the ICM produced a reduced number of new hospital admissions due to AECOPD compared to standard care (3 (IQR=1–6) vs. 5 (IQR=3–8) median admissions respectively, p<0.0001, Table 2) over the period of the study. This resulted in a 38.2% reduction of new hospital admissions among the fe-COPD ICM group (online supplement Table 1; Fig. 2a). Also, the median days of hospitalizations were significantly lower among the ICM group vs. control group (7.4 (IQR=2.95–10.43) vs. 8.65 (IQR=6.0–12.15) days respectively, p=0.002, Table 2). Moreover, the new ER visits were significantly less frequent among the ICM group vs. control group (1 (IQR=0–2) vs. 1 (IQR=0–3) visit respectively, p=0.017, Table 2) with a reduction of 69.7% of the expected ER visits (online supplement Table 1; Fig. 2b). However, there were no statistically significant differences regarding domiciliary hospitalization, hypercapnic respiratory failure admissions, number of positive sputum cultures during admission, number of corticosteroid or antibiotic courses between both groups (p>0.05, Table 2) (Table 3).
Difference in outcomes between integrated care model and standard care.
| Variable | Standard care group(n=132) | ICM group(n=141) | Sig. (p value) |
|---|---|---|---|
| New hospital admissions | 5 (3–8) | 3 (1–6) | <0.0001* |
| New ER visits | 1 (0–3) | 1 (0–2) | 0.017* |
| New domiciliary hospitalization | 0 (0–1) | 0 (0–1) | 0.649 |
| Percentage of hypercapnic respiratory failure admissions (%) | 33.3 (0–79.4) | 45 (0–100) | 0.223 |
| Median days of hospitalization | 8.65 (6.0–12.15) | 7.4 (2.95–10.43) | 0.002* |
| Number of corticosteroid courses | 5 (3–9) | 5 (1–11.25) | 0.733 |
| Number of antibiotic courses | 6 (4–10) | 7 (2–12) | 0.877 |
| Cumulative mortality; n (%) | 78 (59.1) | 70 (49.6) | 0.117 |
| Follow up duration (in months) | 49 (13.5–65) | 31 (14–50) | 0.023* |
ICM: integrated care model, ER: emergency room, fe-COPD: frequent-exacerbator COPD.
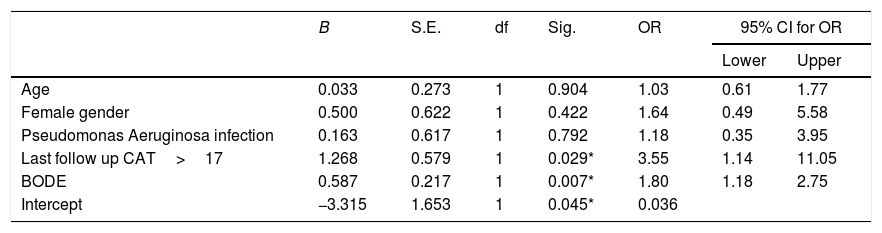
Multivariate analysis in relation to mortality among fe-COPD ICM group.
| B | S.E. | df | Sig. | OR | 95% CI for OR | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Age | 0.033 | 0.273 | 1 | 0.904 | 1.03 | 0.61 | 1.77 |
| Female gender | 0.500 | 0.622 | 1 | 0.422 | 1.64 | 0.49 | 5.58 |
| Pseudomonas Aeruginosa infection | 0.163 | 0.617 | 1 | 0.792 | 1.18 | 0.35 | 3.95 |
| Last follow up CAT>17 | 1.268 | 0.579 | 1 | 0.029* | 3.55 | 1.14 | 11.05 |
| BODE | 0.587 | 0.217 | 1 | 0.007* | 1.80 | 1.18 | 2.75 |
| Intercept | −3.315 | 1.653 | 1 | 0.045* | 0.036 | ||
OR: odd ratio, CAT: COPD assessment test, BODE: “body mass index, airflow obstruction, dyspnea, exercise capacity” index.
When comparing the number of hospital admissions in the intervention group before and after enrolling the ICM program, there was a statistically significant reduction of hospital admissions/year among fe-COPD ICM patients when compared to the admissions 1 year before enrollment (median 1.09 (IQR=0.27–2.57) vs. 3 (IQR=2–5) admissions/year, p<0.0001 respectively; Fig. 3a).
In the multivariate analysis, last follow-up CAT score>17 was the unique independent risk factor associated to two or more new hospital admissions during follow-up among fe-COPD ICM group (OR=7.61, p=0.013, online supplement Table 2). Moreover, admissions 1 year before enrollment in ICM program were not a statistically significant risk factor for further admissions in the same multivariate analysis (OR=1.43, p=0.056, online supplement Table 2).
Health statusThe ICM program had a significant beneficial impact on health status, with a reduction in CAT score from 19 (IQR=14–25) to 15 (IQR=11–20) after 1 year of follow-up (p=0.001, Fig. 3b). Moreover, this effect was maintained or improved over 3 years of follow-up (p=0.01, online supplement Fig. 2); however, mMRC dyspnea scale did not show statistically significant changes over follow-up (p=0.449, online supplement Fig. 3).
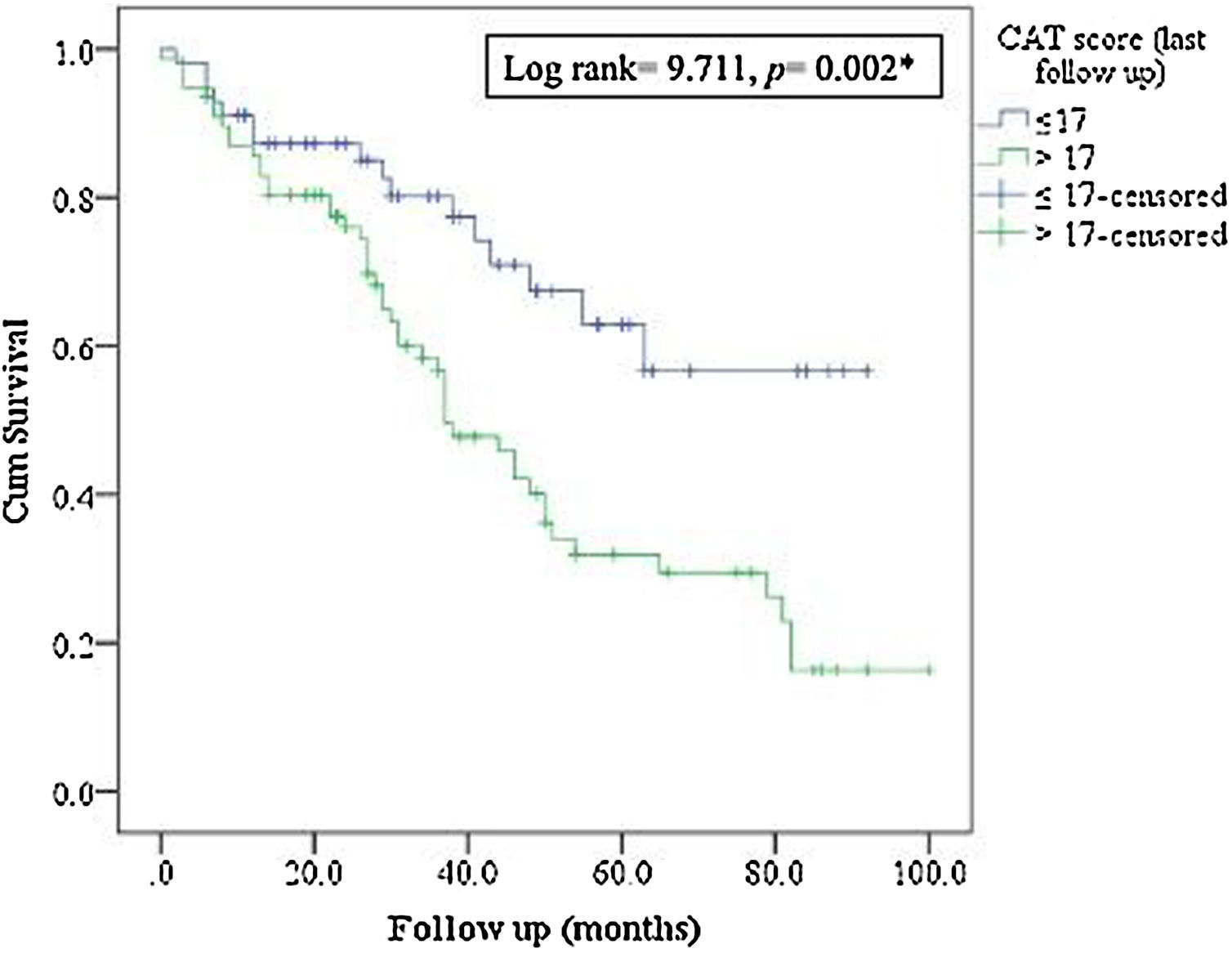
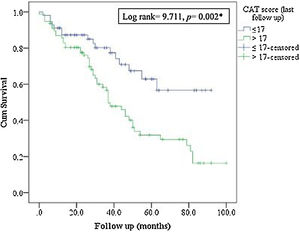
MortalityMore than 50% of patients died during the study period. The ICM had no effect on mortality compared to standard care (p=0.117, Table 1; and online supplement Fig. 4). Female gender, higher baseline mMRC dyspnea scale, Pseudomonas aeruginosa infection, domiciliary oxygen therapy, higher ADO, lower BMI, FEV1 (L), FVC% predicted and FEV1/FVC were risk factors for mortality among the entire fe-COPD population as well as in those included in ICM program using univariate logistic analysis (p<0.05, online supplement Tables 3 and 4 respectively). Further among fe-COPD patients included in the ICM program, last follow-up CAT score>17 and higher BODE index were also significant risk factors for mortality (online supplement Table 4). In a multivariate analysis, last follow-up CAT>17 was the strongest independent risk factor for mortality (OR=3.55, p=0.029, Table 4 online supplement), with survival of 20% after 100 months of follow-up compared to those with CAT≤17 with a survival of 60% (p=0.002, Fig. 4).
DiscussionWe have shown that fe-COPD is associated with impaired health status, high consumption of health resources and high mortality, and that it benefits from a personalized integrated care model that decreases hospital admissions and ER visits when compared to standard care. Moreover, the ICM have a direct impact on the quality of care by improving the health status measured by the CAT score during follow-up although it had no effect in dyspnea nor mortality. This long-term effect on CAT is relevant, since the last CAT>17 is associated to new admissions and mortality.
Previous studiesThe effectiveness of integrated care models has been previously demonstrated in uncontrolled studies. Huertas et al.15 found that a day hospital-based care model for severe COPD patients was associated with a reduction of hospital admission by 71%. Similarly, Jain et al.18 found that there was significant decrease of ER visits and hospitalizations in asthma and COPD patients with frequent exacerbations in a similar ICM. On the other hand, other forms of integrated care using home telemonitoring rather than face-to-face evaluation in frequent COPD exacerbators also reported significant decrease of ER visits, hospital admissions and the number of exacerbations/year.13 Our results are in accordance with these studies; however, our study had the strength of providing a control group of fe-COPD. Further, the previous mentioned studies had an average follow-up of 12 months13,15,18 and maximum of 24 months,15 whereas in the current study, the median follow-up duration was 31 months with maximum of 7 years.
Interpretation of results and clinical implicationsA key objective of COPD management is preventing exacerbations, that have a significant burden on the quality of life especially among severe COPD patients.12,19 According to our results, we believe that enrolment of fe-COPD in an integrated comprehensive program is associated with significant decrease of AECOPD that required ER visits, hospitalization, and, in case patients needed to be admitted, a reduction of hospitalization days.
Several factors may explain the effectiveness of ICM in the current study: (1) the flexibility of the program that enabled the patient to access to medical care through a direct telephone call and so the early detection of exacerbations and subsequent management; (2) rapid arrangement of follow-up visits after phone calls for evaluation of patients after exacerbations; (3) the health care is provided by the same team who are familiar with the patients’ characteristics; (4) the program is conducted in a tertiary hospital with all facilities for rapid tracking and evaluation of the patients; (5) the care model is coordinated with Primary Care and other hospital departments such as Hospital-at-home, Rehabilitation, Nutrition or social assistant. All of these factors also increased the adherence of the patients to the ICM visits and the given instructions which positively enhanced the impact of the program.
However, despite the impact of this model of care on hospital utilization, we did not find differences in mortality when comparing to standard care. This could be explained on the basis that fe-COPD patients enrolled in the ICM were more severe in terms of lower FEV1 and higher basal mMRC dyspnea scale compared to control group (although there was a similar proportion of GOLD D and C in both groups as well as ADO index). We found that ADO index was a significant risk factor of mortality in the entire fe-COPD population that was not superior than mMRC dyspnea scale (online supplement Table 3). Other studies have shown that basal mMRC dyspnea scale is a good predictor of mortality and AECOPD.20 mMRC was also a good predictor of mortality in our cohort that was not influenced by the ICM. However, dyspnea is a complex symptom that can be explained by different pathophysiological mechanisms, especially in a more severe and frequent-exacerbator population like ours. When we evaluated a multidimensional score such as the BODE index, an important predictor of survival among stable COPD irrespective of their ABCD grouping,21 we found that it was a significant risk factor of mortality. However, in our population of fe-COPD receiving ICM, BODE index was not superior to CAT score for the prediction of mortality. CAT is a simple questionnaire that has been validated as a multi-dimension assessment tool for COPD patients and can be used for the evaluation of treatment response.22 Further, there was a significant decrease of CAT score after 1 year and 3 years of follow-up among fe-COPD patients enrolled in the program denoting better control of the disease and improving health status. Not surprising, we found that last follow-up CAT score>17 was the strongest predictor for new admissions and mortality among fe-COPD. CAT score>17 has been used as cutoff value for the identification of poor health status and moderate-severe exacerbations leading to death.20,23 Rassouli et al.24 also reported a significant positive association between CAT score changes and risk of AECOPD. In our understanding, this finding may have important prognostic implications since the ICM have a direct impact on CAT.
The high mortality rate observed in the studied COPD population could be explained by the severity of the underlying COPD disease especially FEV125, but also by patients’ demographics. Morevover, COPD is associated to a persistent underlying systemic inflammatory process that could be a mediator of extra-pulmonary complications and a risk factor for mortality.26,27 Moreover, still there is a percentage of COPD who die during their sleep or in whom death was unexpected.28
Interestingly, we found that female gender was associated to mortality in our fe-COPD cohort. Ringbaek et al.29 found that women with COPD had double risk for mortality compared to men. Further, Stolz et al.30 found that females with COPD were at greater risk for moderate-to-severe AECOPD than males. Moreover, Pseudomonas aeruginosa infection was also a significant risk factor for mortality but other bacterial infections were not. Pseudomonas aeruginosa infection was shown to be a predictor of 3-year mortality after hospitalization for AECOPD31 and in outpatient stable COPD patients.32 However, age, gender or Pseudomonas aeruginosa infection were not independent risk factors in the multivariate analysis among fe-COPD included in the ICM program, which we think that can be explained by the interactions among all these factors on the severity of the disease.
Study limitationsThe current study has some limitations. Firstly, there could be a selection bias since the control group was not randomly assigned. However, the population enrolled in the ICM was more severe than the control group which reinforces the conclusions. Moreover we thought that it was unethical to choose a randomized control group when we have a specialized program of care for this group of patients. Secondly, the data of hospitalizations, AECOPD and treatments of the control group were obtained from the recorded files of the hospital; however, we assumed that there was no significant bias in the analysis as all the patients were recruited and followed up in the same hospital. Thirdly, we did not analyze the impact of the ICM in terms of costs. The saving on hospital admissions and ER visits should be counterbalance with the cost of having a structure with a full-time nurse and a respiratory specialist on demand. Since the facilities and personnel were already in place and were reallocated to exert this function, we anticipate that the cost/benefit was high for our institution, although detailed economic analysis is needed. Previous studies considered telemonitoring of COPD had significant reduction of total costs for COPD management.33,34 Fourth, the current program was applied in a single center and all the patients were living in the same geographical area; however, this fact improved the adherence to the program, so enhanced better control of the patients. Lastly, we did not analyze social factors and physical activity as risk factors among our studied population. Physical activity level is considered as an important factor associated with mortality and exacerbations in COPD population,35 however, our ICM program is integrated and personalized offering rehabilitation and social support to our patients as part of their usual care.
ConclusionsA comprehensive and personalized integrated care model for fe-COPD patients effectively decreased ER visits and hospital admissions due to exacerbations and improved steadily health status measured by CAT. Since this model of care have no impact on mortality, we demonstrated that our approach helped frequent-exacerbator patients not to live longer but to live better.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestNone.