The impact of obstructive lung disease (OLD) and emphysema on lung cancer (LC) mortality in patients undergoing LC screening is controversial.
MethodsPatients with spirometry and LC diagnosed within the first three rounds of screening were selected from the National Lung Screening Trial (NLST) and from the Pamplona International Early Lung Cancer Detection Program (P-IELCAP). Medical and demographic data, tumor characteristics, comorbidities and presence of emphysema were collected. The effect of OLD and emphysema on the risk of overall survival was assessed using unadjusted and adjusted Cox models, competing risk regression analysis, and propensity score matching.
ResultsData from 353 patients with LC, including 291 with OLD and/or emphysema and 62 with neither, were analyzed. The median age was 67.3 years-old and 56.1% met OLD criteria, predominantly mild (1: 28.3%, 2: 65.2%). Emphysema was present in 69.4% of the patients. Patients with OLD and/or emphysema had worse survival on univariate analysis (HR: 1.40; 95% CI: 0.86–2.31; p=0.179). However, after adjusting for LC stage, age, and sex, the HR was 1.02 (95% CI: 0.61–1.70; p=0.952). Specific LC survival between both groups showed an adjusted HR of 0.90 (95% CI: 0.47–1.72; p=0.76). Propensity score matching found no statistically significant difference in overall survival (HR: 1.03; 95% CI: 0.59–1.9; p=0.929).
ConclusionThe survival of LC patients diagnosed in the context of screening is not negatively impacted by the coexistence of mild OLD and/or emphysema.
Lung cancer (LC) is the leading cause of cancer-related mortality worldwide.1 Smoking is not only the primary risk factor for LC, but increases the risk of other respiratory diseases such as chronic obstructive pulmonary disease (COPD) and emphysema,2 which in turn are strongly associated with the development of LC.3–7 Lung cancer screening is effective in reducing LC mortality,8–10 but there is a growing concern about the risks of screening patients with coexisting chronic lung disease.11
The impact of COPD and emphysema on mortality in patients with LC remains controversial. Some studies have reported a worse prognosis attributed to post-treatment complications.12–14 However, improvements in bronchodilator treatment,13,15 physical therapy,16 and even advances in thoracic surgery and radiation therapy17 have challenged these findings in other studies. Moreover, treatment of early-stage LC in patients with coexisting COPD or emphysema may even result in spirometric improvements due to a reduction of lung volume.18–22
Evidence regarding the impact of these pulmonary diseases on LC mortality in lung cancer screening programs is limited, and generally based on secondary analyses of cohorts with limited sample size and/or underpowered statistical analysis.23 Due to the large proportion of patients with COPD and/or emphysema participating in these programs,24 there is a need for larger studies focused on the impact of these respiratory comorbidities on survival of patients who are diagnosed with LC in the context of screening. Our study focuses on patients who were screened for LC but also underwent lung function testing from the ACRIN sub-cohort of the National Lung Screening Trial (NLST) and the Pamplona (Spain) sub-cohort of the International Early Lung Action Program (P-IELCAP). To avoid confounding factors and to increase precision, competing risks and propensity score matching analysis were used.
MethodsTrials OversightThe National Lung Screening Trial (NLST) was a randomized trial comparing screening for lung cancer using LDCT with chest radiography. Participants were invited to undergo three screening rounds (T0, T1, T2) at 1-year intervals. The NLST enrolled participants between August 2002 and September 2007, and followed through December 2009. The NLST was approved by the institutional review board at each of the 33 participating medical institutions. Details about the study protocol, participant follow-up, medical-record abstraction, LDCT and lung function test characteristics are detailed in the original study.9
The Pamplona sub-cohort of the International Early Lung Cancer Action Program (P-IELCAP) is a consolidated lung cancer screening program that began in 2000 in Spain, and has screened more than 5000 individuals at risk for LC. The ongoing program offers ongoing annual LDCT and pulmonary function testing for all participants. Details of the protocol have been described previously.25
Study PopulationThe NLST included current or former smokers (abstinence <15 years) with ages ranging from 55 to 74 years at the time of randomization, and a smoking history of at least 30 pack-years. P-IELCAP enrolled adults ≥40 years-old, with a smoking history of more than 10 pack-years.
A total of 53,452 individuals were enrolled in the NLST and 26,722 were randomly assigned to screening with low-dose computed tomography (LDCT). LC was diagnosed in 1089 individuals in the LDCT arm, of which 790 were found during the three rounds of screening (T0–T2). Screening by the American College of Radiology Imaging Network (ACRIN cohort), included spirometry testing at enrollment.26 For the purpose of our study, we selected participants who were diagnosed with LC during the first three rounds of screening and had spirometry performed during the study, yielding a final cohort of 235 NLST patients.
In the P-IELCAP cohort, a total of 126 patients were diagnosed with LC between February 2001 and September 2021. For the purpose of this study, we selected 118 patients who had valid lung function testing.
Population CharacteristicsDemographic characteristics and comorbidities were recorded at baseline in both cohorts. The presence of emphysema was visually determined on the LDCT. Prebronchodilator spirometry was performed at baseline and OLD was defined by the presence and severity of airway limitation based on Global Initiative for Chronic Obstructive Lung Disease criteria (FEV1/FVC<70, grades 1–4).27 Patients were classified into two groups, i.e., patients with OLD and/or emphysema (OLD/emphysema), and patients with normal lung function and no emphysema on LDCT. The latter group were classified as “smokers without lung disease.”
Tumor characteristics were also recorded for all participants with lung cancer. Disease stage was determined according to the seventh edition of the Cancer Staging Manual of the American Joint Committee on Cancer, and histology was classified according to the International Classification of Diseases for Oncology, 3rdEdition (ICD-O-3).28
StatisticsDescriptive statistics were used to summarize the characteristics of the study population. Absolute and relative frequencies were used for qualitative data. The means (SD) and medians (25th–75th percentile) were estimated for quantitative variables with normal and non-normal distributions, respectively. Normal distributions were assessed by the Shapiro–Wilk test. Baseline data were compared between study groups (healthy smokers or COPD and/or emphysema) using the Wilcoxon signed-rank test for continuous variables and the chi-square test (or Fisher's exact test when the expected frequencies were less than five in some cells) for qualitative variables. Furthermore, baseline data were compared between disaggregated groups (smokers without lung disease, OLD and emphysema) using ANOVA (or Kruskal–Wallis test for variables with non-normal distribution) for continuous variables. The effect of the presence of OLD and/or emphysema on the risk of overall mortality was assessed using unadjusted and adjusted Cox model. Age, sex and TNM stage were included in the model as confounding factors. Additionally, risk of cancer mortality was evaluated using Fine–Gray's model considering mortality from other causes as competing risk.29 The same survival analysis was performed for disaggregated study groups. The dose–response relationship between pulmonary function parameters (forced expiratory volume in the 1st second [FEV1] and forced vital capacity [FVC]) with overall mortality risk in OLD population was assessed using Cox proportional hazard model adjusted for confounding factors (age, sex and cancer stage). Time elapsed from cancer diagnosis to event was used in survival analyses. Finally, sensitivity analysis with the same survival analysis previously described was applied in a population matched for sociodemographic and clinical characteristics. The matching process, using a 2:1 ratio, was performed using a nearest neighbors matching with a propensity score caliper distance of 0.1 to select matched patients between groups. Matching processes were performed to enable comparability between study groups. A standardized mean difference (SMD) between groups of less than 0.1 was defined as optimal quality match. Matching processes included age, sex, TNM stage. R statistical software, version 4.0.1 (R Project for Statistical Computing) was used for all analyses.
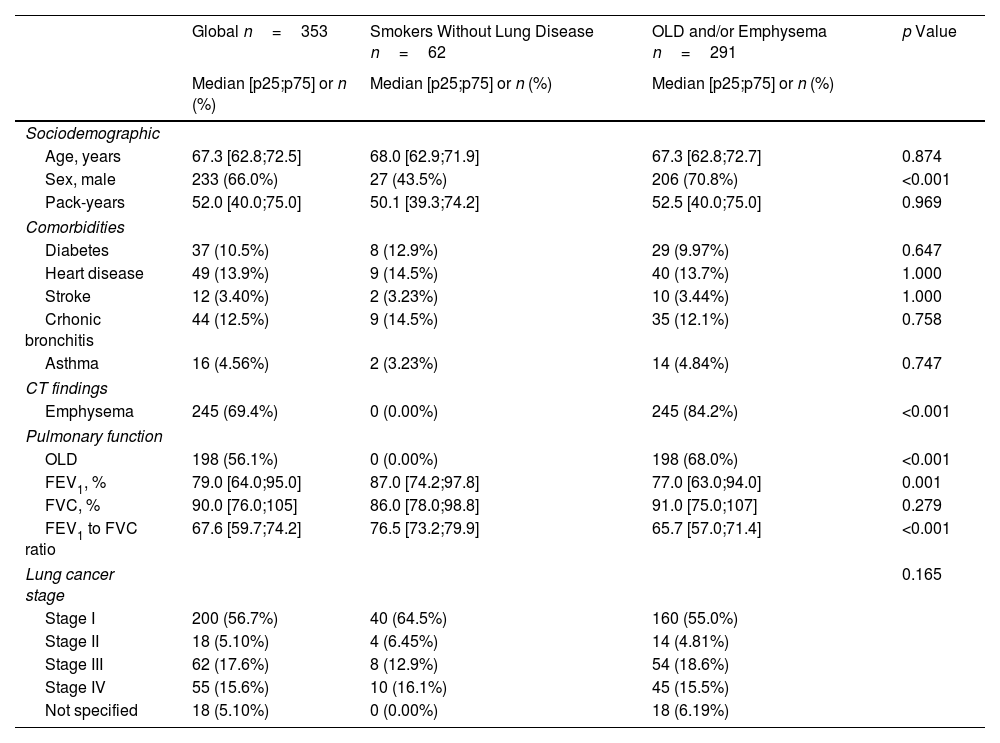
ResultsBaseline Clinical Characteristics, Lung Function and LC StageThe characteristics of the study population are shown in Table 1. A total of 353 patients with LC from both cohorts were included in the analysis, of whom 291 had OLD and/or emphysema and 62 were classified as “smokers without lung disease”. The median (p25;p75) age was 67.3 years (62.8;72.5). The cohort was predominantly male (66%) and heart disease (13.9%), chronic bronchitis (12.5%) and diabetes (10.5%) were the most common comorbidities. More than a half of the population (56.1%) met OLD criteria, with a median FEV1 of 65.5% (60.2;80.8). These patients had predominantly mild severity of airway obstruction (1: 28.3%, 2: 65.2%), with only 6.5% in stage 3, and none in stage 4. Emphysema was present in 69.4% of the entire cohort. Patients in the OLD/emphysema group were more likely males (70.8% vs 43.5%; p<0.001) with worse lung function (median of FEV1 of 77% (63;94) vs 87% (74.2;97.8); p<0.001). No differences in other comorbidities between the groups were seen (Table 1). When compared to smokers without lung disease, LC in the OLD/emphysema group tended to be diagnosed in more advanced stages (29% vs 36%, respectively, diagnosed in stages 3–4), but the difference did not reach statistical significance (Table 1). Sociodemographic and clinical characteristics stratified by lung function and the presence of emphysema (three groups) are shown in Table S1.
Sociodemographic and Clinical Characteristics According to Presence of OLD and/or Emphysema.
| Global n=353 | Smokers Without Lung Disease n=62 | OLD and/or Emphysema n=291 | p Value | |
|---|---|---|---|---|
| Median [p25;p75] or n (%) | Median [p25;p75] or n (%) | Median [p25;p75] or n (%) | ||
| Sociodemographic | ||||
| Age, years | 67.3 [62.8;72.5] | 68.0 [62.9;71.9] | 67.3 [62.8;72.7] | 0.874 |
| Sex, male | 233 (66.0%) | 27 (43.5%) | 206 (70.8%) | <0.001 |
| Pack-years | 52.0 [40.0;75.0] | 50.1 [39.3;74.2] | 52.5 [40.0;75.0] | 0.969 |
| Comorbidities | ||||
| Diabetes | 37 (10.5%) | 8 (12.9%) | 29 (9.97%) | 0.647 |
| Heart disease | 49 (13.9%) | 9 (14.5%) | 40 (13.7%) | 1.000 |
| Stroke | 12 (3.40%) | 2 (3.23%) | 10 (3.44%) | 1.000 |
| Crhonic bronchitis | 44 (12.5%) | 9 (14.5%) | 35 (12.1%) | 0.758 |
| Asthma | 16 (4.56%) | 2 (3.23%) | 14 (4.84%) | 0.747 |
| CT findings | ||||
| Emphysema | 245 (69.4%) | 0 (0.00%) | 245 (84.2%) | <0.001 |
| Pulmonary function | ||||
| OLD | 198 (56.1%) | 0 (0.00%) | 198 (68.0%) | <0.001 |
| FEV1, % | 79.0 [64.0;95.0] | 87.0 [74.2;97.8] | 77.0 [63.0;94.0] | 0.001 |
| FVC, % | 90.0 [76.0;105] | 86.0 [78.0;98.8] | 91.0 [75.0;107] | 0.279 |
| FEV1 to FVC ratio | 67.6 [59.7;74.2] | 76.5 [73.2;79.9] | 65.7 [57.0;71.4] | <0.001 |
| Lung cancer stage | 0.165 | |||
| Stage I | 200 (56.7%) | 40 (64.5%) | 160 (55.0%) | |
| Stage II | 18 (5.10%) | 4 (6.45%) | 14 (4.81%) | |
| Stage III | 62 (17.6%) | 8 (12.9%) | 54 (18.6%) | |
| Stage IV | 55 (15.6%) | 10 (16.1%) | 45 (15.5%) | |
| Not specified | 18 (5.10%) | 0 (0.00%) | 18 (6.19%) | |
Abbreviations: OLD, obstructive lung disease; CT, computerized tomography; FEV1, forced expiratory volume in 1s; FVC, forced vital capacity.
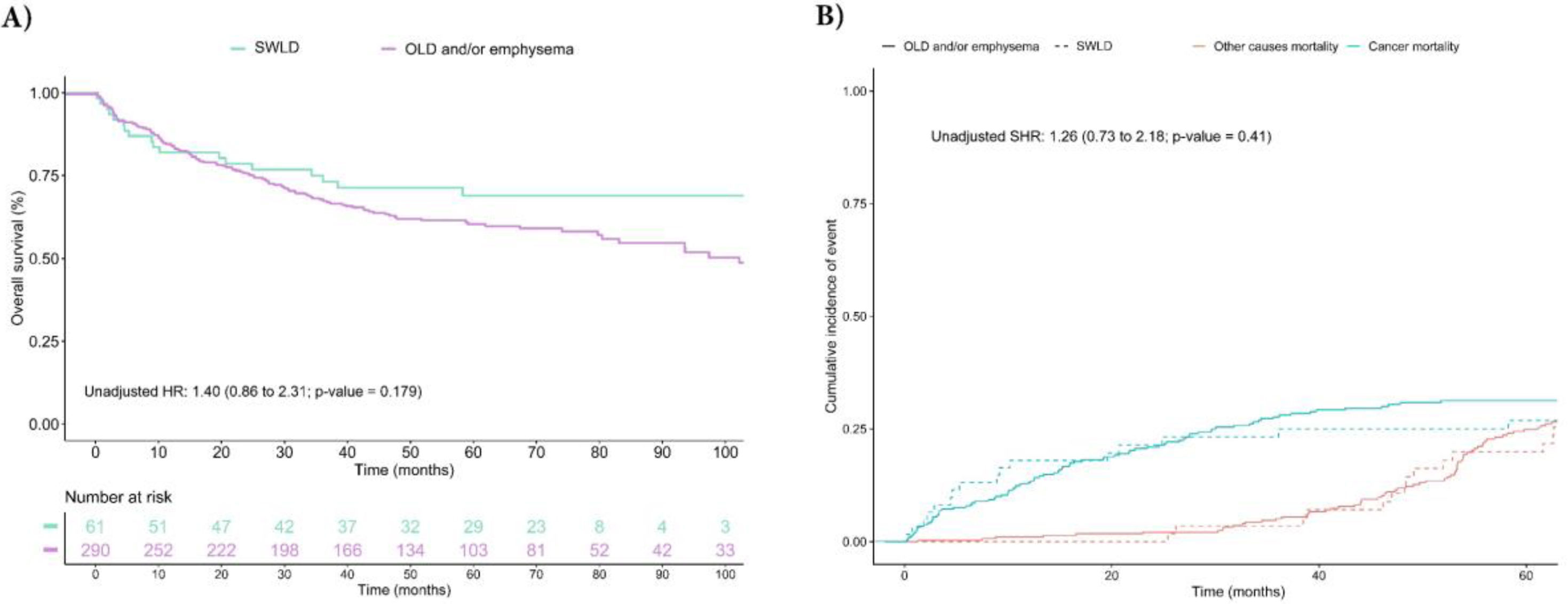
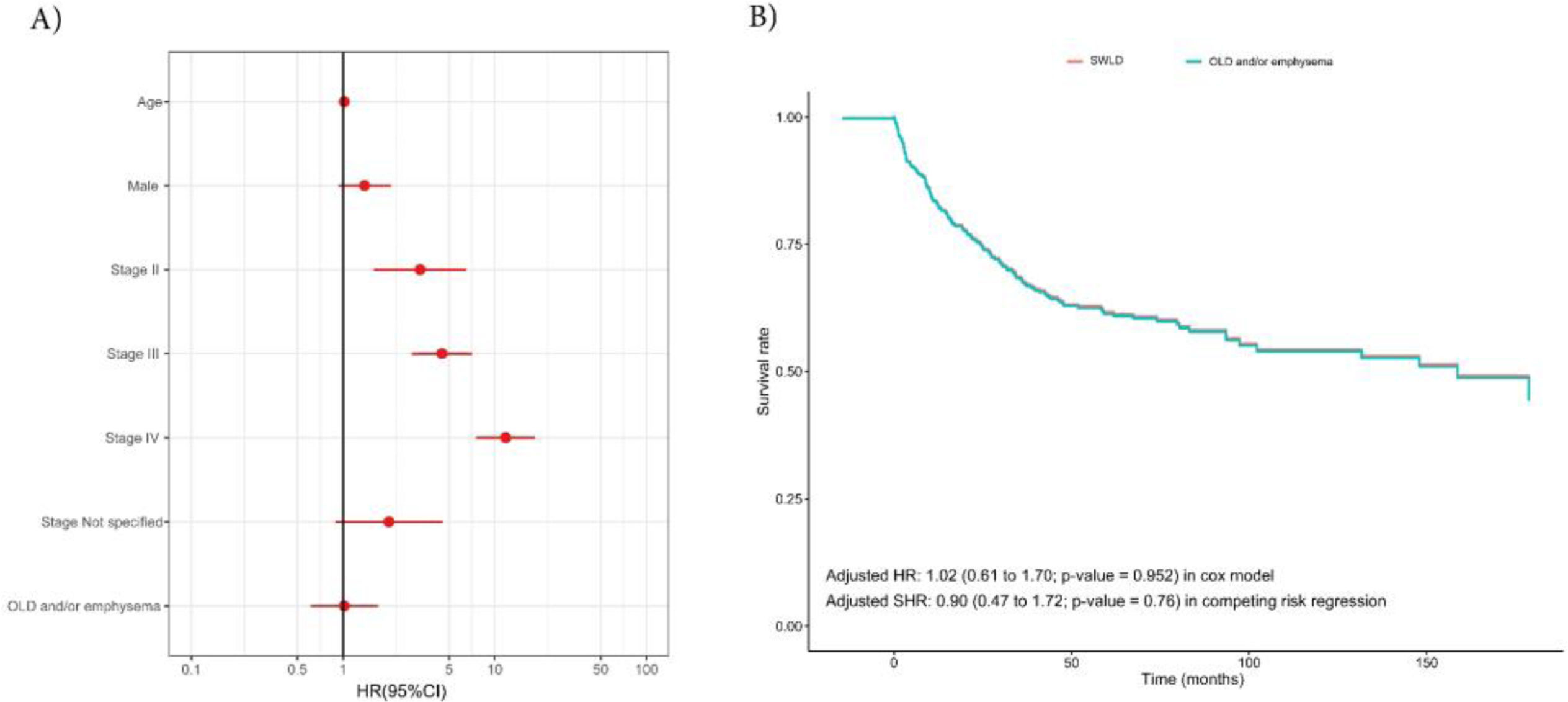
Fig. 1 and model 1 of Table S2 show unadjusted survival analysis of mortality and specific LC survival between the groups of smokers without lung disease and COPD/emphysema. Individuals in the OLD/emphysema group had the worst survival rate with a HR (95% CI) of 1.40 (0.86–2.31; p=0.179) in Cox model, and a SHR of 1.26 (0.73–2.18; p=0.41) in competing risk regression. However, no difference was observed between groups when adjusting for the most important confounding factors (age, sex and LC stage) in Cox and competing risk regression with HRs of 1.02 (95% CI: 0.61–1.70; p=0.952) and 0.90 (0.77–1.72; p=0.76), respectively (Fig. 2 and model 1 of Table S2). Furthermore, in patients with OLD we did not observe a dose–response relationship after adjusting for confounding factors between pulmonary function (FEV1 and FVC) and overall survival (Fig. S1). Similar results were obtained when the unadjusted and adjusted survival models were performed in the OLD and emphysema groups separately (model 2 of Table S2 and Figs. S2–S4).
Unadjusted survival analysis for overall mortality and cancer mortality according to study groups. (A) The Kaplan–Meier curve and unadjusted/adjusted HR (95% CI) estimated using Cox regression model. Age, sex and TNM stage were included as adjustment variables. (B) Cumulative Incidence curves of events for competing risks analysis and unadjusted/adjusted subdistribution hazard (95% CI) for cancer mortality. Abbreviations: SWLD, smokers without lung disease; OLD, obstructive lung disease; HR, hazard ratio; SHR, subdistribution hazard ratio; CI, confidence interval.
Adjusted survival models according to the presence of OLD and/or emphysema. (A) Hazard ratios (95% CI) of multivariable Cox model. (B) Adjusted survival curves for Cox proportional hazards model according to the presence of OLD and/or emphysema. Abbreviations: SWLD, smokers without lung disease; OLD, obstructive lung disease; HR, hazard ratio; SHR, subdistribution hazard ratio; CI, confidence interval.
To corroborate these results, propensity score matching was used to enable comparisons between study groups. After the matching process (1:2, including: age, sex and LC stage), a total of 60 smokers without lung disease were matched with 121 patients with OLD and/or emphysema. Sociodemographic and clinical characteristics of this matched population are shown in Table S3. After the matching process, the groups showed covariate balance in confounding factors, highlighting the importance of these analyses to make the groups more homogeneous and comparable (Fig. S5-A). Finally, the Kaplan–Meier curves according to the study groups showed no statistically significant differences in overall survival with a HR of 1.03 (95% CI: 0.59–1.9; p=0.929; Fig. S5-B).
DiscussionOur results show that LC mortality in the context of screening is not influenced by the presence of mild OLD and/or emphysema as demonstrated by competing risk and propensity score matching of two lung cancer screening cohorts (NLST and P-IELCAP).
These results assuage concerns regarding the impact of OLD and emphysema on the risks and benefits of lung cancer screening.11,30 Whether more severe forms of OLD or emphysema can affect screening outcomes has yet to be determined, since most individuals offered screening have mild disease given the need for being candidates for resection.23,31,32 This is crucial, mounting evidence shows that LC is one of the most important causes of death in patients with early stages of COPD.33–35 Previous studies have shown that in patients undergoing screening, the presence of COPD doubles the incidence of LC, reduces the risk of overdiagnosis, and is associated with a more favorable stage shift and a reduction in mortality.23,26 Our results, with a fairly larger number of LC cases and using robust statistical analyses, strengthen these findings by focusing on patients with LC diagnosed in the context of annual LC screening.
Several previous studies point to COPD and emphysema as contributing factors to worse overall survival and disease-free survival (DFS) in patients with LC.12–14 Most of them were small retrospective cohorts of patients with resected LC.12–14 However, the mild COPD group in these studies showed similar DFS compared to the non-COPD group14 as shown in our study. Additionally, a metanalysis of 27 studies13 revealed that the presence of COPD and emphysema was associated with poorer overall survival (HR: 1.17; 95% CI: 1.10–1.25 and HR: 1.66; 95% CI:1.25–2.22, respectively). Nevertheless, some aspects should be considered before drawing conclusions, such as the high heterogeneity found between the studies (I2=78%) and the fact that COPD diagnosis was self-recorded or identified from the medical record without spirometry in more than a half of the cohorts. More importantly, none of these studies were performed in the context of lung cancer screening programs. The limited research conducted within screening contexts indicates a higher incidence and mortality rate of lung cancer (LC), as well as a greater frequency of diagnoses at advanced stages in patients with obstructive lung disease (OLD) and/or emphysema.36,37 The fact repeatedly observed in these studies (and in ours) that patients with these underlying lung diseases have a more aggressive LC12,26 appears not to affect survival in mild OLD patients undergoing lung cancer screening programs.
As many as 40–66% of LCs detected in established lung cancer screening programs and screening trials occur in individuals with COPD and/or emphysema,24,38,39 our study demonstrates that these patients do not exhibit worse survival despite underlying pulmonary comorbidities. As mentioned before, tumor stage remains the only variable consistently associated with a worse prognosis. Additionally, it has been observed that the treatment of early LC stages in patients with COPD and emphysema can minimize pulmonary function loss after surgery or sometimes even improve it. This occurs more often in patients with emphysema when the LC is located in an emphysematous area (as it usually is) resulting in lung volume reduction.18–22 For these reasons, patients with mild COPD and emphysema should not be excluded from screening programs. There is an increasing general recognition of this fact, even as an implicit recommendation in the new GOLD guidelines for the management of patients with COPD.2
This study has several limitations. Firstly, it is a retrospective review of a prospective trial, with a sub-selection of individuals due to the lack of lung function on the entire cohort. However, the included cohort was fairly large and had been assigned to have lung function tests a priori, which limits bias due to selection. Secondly, we labeled patients with irreversible airflow obstruction and OLD and not COPD because the definition of the latter should include symptoms and we do not have access to that information. For this reason, these findings may not be generalizable to all patients with COPD. Moreover, lung function is not expressed using currently accepted Z-scores. Thirdly, only prebronchodilator spirometry lung function was done and this is known to overestimate the prevalence of “COPD”.40 It is possible that a more rigorous selection of patients with COPD, would have resulted in a significant influence of the presence of OLD on LC specific survival. Finally, emphysema was determined visually by radiologists and no quantification or grading of the severity is available. A validation of the diagnosis of emphysema (or its quantification by software) in the cohort has not been performed and the effect of different grades of severity cannot be analyzed.
In conclusion, the outcomes of patients with LC diagnosed in the context of lung cancer screening with an annual LDCT is not affected by the presence of mild OLD and/or emphysema. In clinical practice, these patients should be considered candidates for lung cancer screening programs.
Role of the Funding SourceFunding agencies were not involved in study design, data collection nor analysis, decision to publish, or preparation of the manuscript.
FundingThis work was supported in part by a grant [RD12/0036/0062 and RD12/0036/0040] from Red Tematica de Investigacion Cooperativa en Cancer, Instituto de Salud Carlos III, the Spanish Ministry of Economy and Competitiveness and European Regional Development Fund “Una manera de hacer Europa.” It was also supported by grants PI04/2404, PI07/0792, PI10/01652, and PI11/01626 from the Instituto de Salud Carlos III, Ministry of Economy and Competitiveness, Government of Spain.
Authors’ ContributionsJ.J. Zulueta is the guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article. JG, JPdT, IdB, LMS, JPW, FB, and JJZ were responsible for conception and design, analysis and interpretation, and drafting of the manuscript for important intellectual content.
Conflict of InterestsLMS has received consultancy fees for participating in advisory boards for Sabartech, the Lung Ambition Alliance, and Serum; he also received fees for speaking activities from Astra Zeneca, Roche, and Merck, Sharp & Dohme Corporation.
JJZ has received consultancy fees for participating in advisory boards for Median Technologies and American Heart Technologies; he is a shareholder of VisionGate, Inc.
JPW has received honorarium from PPD, Banook, Sanofi and grants from Sanofi, Regeneron, Axella and Arlnold Consultants.
None declared (JG, JPdT, IdB, MMO, FB).