In patients with non-small cell lung cancer (NSCLC) and normal mediastinal imaging tests, centrally located tumors have greater occult mediastinal involvement. Clinical guidelines, therefore, recommend invasive mediastinal staging in this situation. However, definitions of centrality in the different guidelines are inconsistent. The SEPAR Thoracic Oncology area aimed to evaluate the degree of familiarity with various concepts related to tumor site among professionals who see patients with NSCLC in Spain.
MethodsA questionnaire was distributed to members of Spanish medical societies involved in the management of NSCLC, structured according to the 3 aspects to be evaluated: 1) uniformity in the definition of central tumor location; 2) uniformity in the classification of lesions that extend beyond dividing lines; and 3) ability to delineate lesions in the absence of dividing lines.
ResultsA total of 430 participants responded. The most voted definition of centrality was «lesions in contact with hilar structures» (49.7%). The lines most often chosen to delimit the hemithorax were concentric hilar lines (89%). Most respondents (92.8%) classified tumors according to the side of the dividing line that contained most of their volume. Overall, 78.6% were able to correctly classify a central lesion in the absence of dividing lines.
ConclusionsIn our survey, the most widely accepted definition of centrality is not one of the proposals specified in the clinical guidelines. The results reflect wide variability in the classification of tumor lesions.
En pacientes con cáncer de pulmón de célula no pequeña (CPCNP) y mediastino normal, en pruebas de imagen, los tumores de localización central presentan mayor afectación mediastínica oculta. Por ello las guías clínicas recomiendan estadificación mediastínica invasiva en esta situación. No obstante, las definiciones de centralidad son poco uniformes entre guías. Desde el área de oncología torácica de la SEPAR se propuso evaluar el grado de familiaridad con varios conceptos relacionados con la localización tumoral entre profesionales que atienden pacientes con CPCNP en nuestro territorio.
MétodosSe envió una encuesta a miembros de sociedades médicas nacionales implicadas en el manejo del CPCNP. La encuesta se estructuró en tres aspectos a evaluar: 1) uniformidad en la definición de localización tumoral central; 2) uniformidad en la clasificación de lesiones que sobrepasan líneas divisorias y 3) capacidad para delimitar lesiones en ausencia de líneas divisorias.
Resultados430 participantes respondieron. La definición de centralidad más votada fue «lesiones en contacto con las estructuras hiliares» (49,7%). Las líneas más escogidas para delimitar el hemitórax fueron líneas concéntricas al hilio (89%). La mayoría (92,8%) consideró los tumores según en qué lado de la línea divisoria se encontrase la mayor parte de su volumen. Un 78,6% fue capaz de catalogar correctamente una lesión central en ausencia de líneas divisorias.
ConclusionesEn nuestra encuesta, la definición de centralidad más aceptada no es ninguna de las propuestas en las guías clínicas. Los resultados reflejan amplia variabilidad para clasificar lesiones tumorales.
In patients with non-small cell lung carcinoma (NSCLC), mediastinal staging is essential for determining both prognosis and optimal treatment. The initial study of the mediastinum is based on computed tomography (CT) and positron emission tomography (PET), both separately and in combination (PET/CT). However, imaging techniques have diagnostic limitations and in some cases a negative result may hide mediastinal nodal involvement. More specifically, 3 clinical situations have been associated with a high rate of false-negative PET results: tumors larger than 3 cm in diameter; N1 involvement in imaging tests; and centrally located tumors. For this reason, evidence-based clinical guidelines for presurgical mediastinal staging of NSCLC released by the European Society of Thoracic Surgeons (ESTS),1 the American College of Chest Physicians (ACCP),2 and the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR)3 recommend invasive mediastinal staging not only in patients with pathological mediastinal findings on CT and/or PET, but also in individuals with normal mediastinum in any of the 3 clinical situations described. While the measurement of tumors larger than 3 cm can be objective and is relatively easy to perform, the determination of centrally located tumors is very controversial as there is no uniform definition of central tumor location. The ESTS refers to “tumors located in the inner two thirds of the lung”, whereas the ACCP refers to “tumors located in the inner third of the hemithorax”, and SEPAR refers to “centrally located tumors, usually in contact with the mediastinum”. Moreover, other definitions of central location described in scientific studies (such as that of Gómez-Caro et al.)4 or in guidelines from other societies (for example, as an exclusion criterion for stereotactic radiation therapy)5 have only added to the confusion. This lack of uniformity leads to wide variability in clinical practice among professionals responsible for managing patients with NSCLC. In this regard, a survey of 218 thoracic surgeons and pulmonologists was recently conducted in the United States.6 The results showed that professionals responsible for the care of NSCLC patients used a wide range of criteria for defining central lesions. Given this lack of uniform criteria in the literature and among the different societies, the SEPAR Thoracic Oncology area identified a need for a cross-sectional study to assess the situation in Spain. The aim of our study, then, was to evaluate the degree of familiarity with various concepts related to central tumor location among professionals who treat NSCLC patients in Spain. More specifically, the aim was to evaluate: 1) uniformity in the definition of central tumor location; 2) uniformity in the classification of lesions that extend beyond dividing lines; and 3) respondents’ ability to delineate lesions in the absence of dividing lines.
MethodsA freely accessible questionnaire composed of multiple-choice questions, with or without chest CT images, was sent by e-mail to all members of the Thoracic Oncology area of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR), the Spanish Society of Thoracic Surgery (SECT), the Spanish Lung Cancer Group (GECP), and the Spanish Society of Cardiothoracic Imaging (SEICAT).
QuestionnaireThe survey link opened on June 4 and closed on September 15, 2019. The questionnaire was structured in 3 blocks:
- 1)
Definition of centrality (Fig. 1): this block evaluated the uniformity of criteria defining centrality.
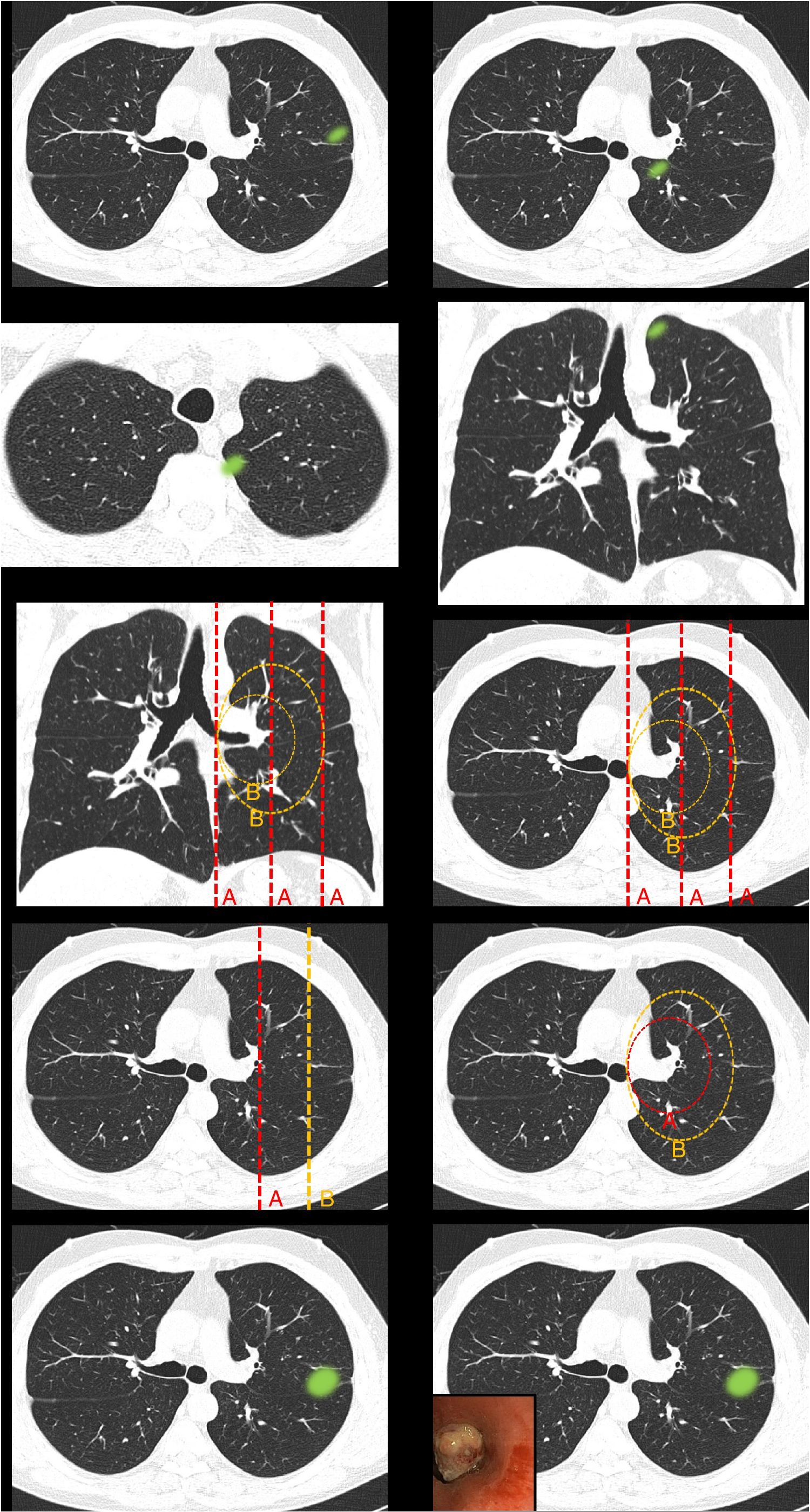
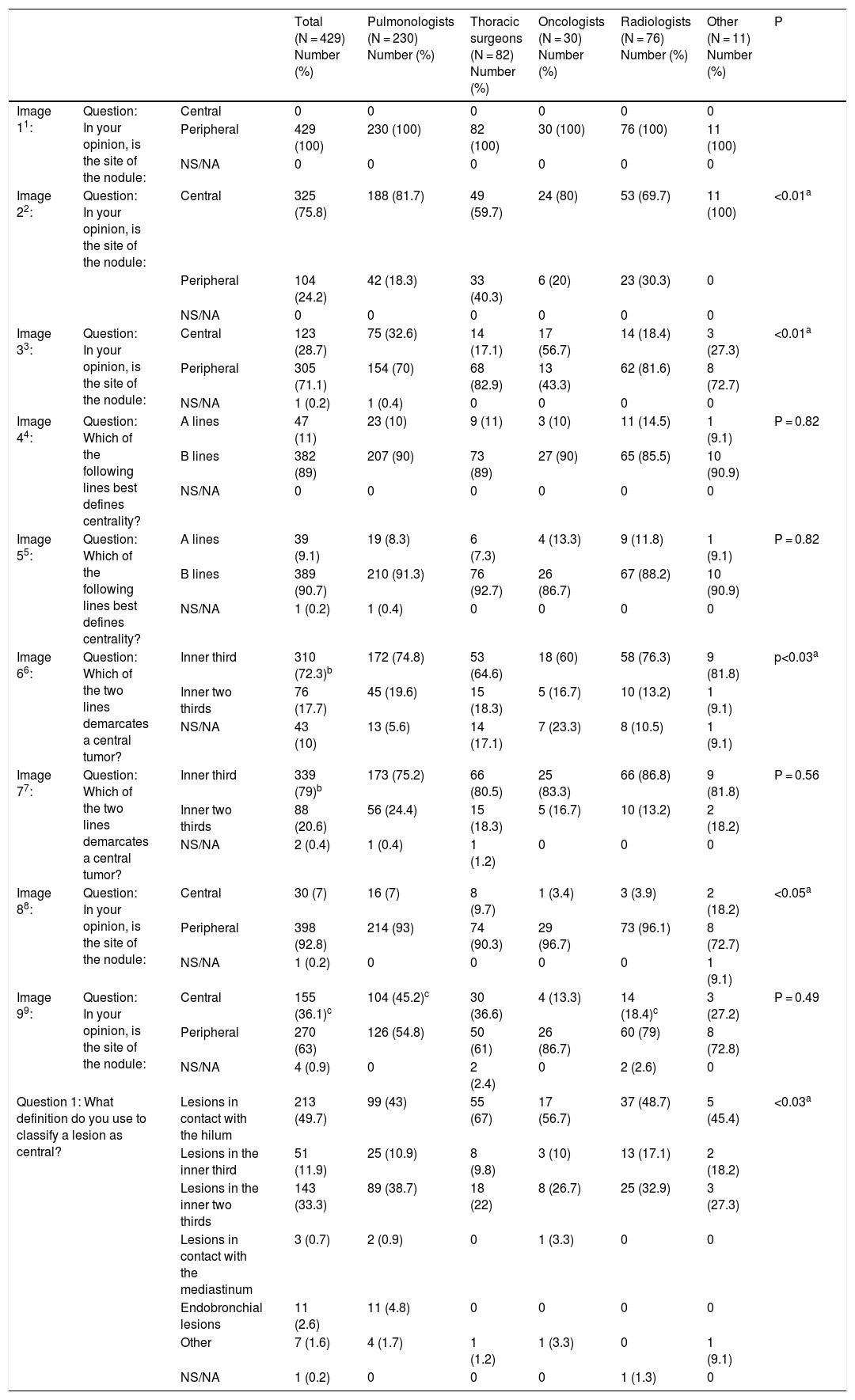
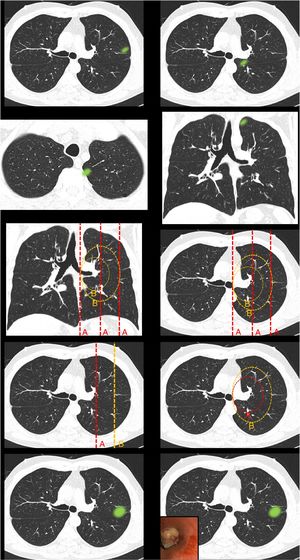
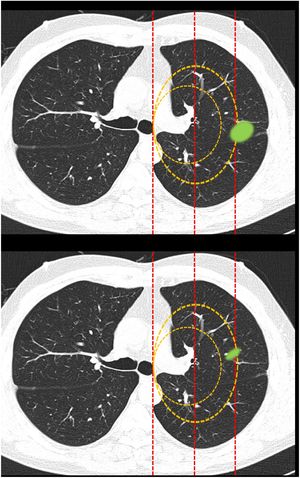
Fig. 1.This block evaluated the uniformity of criteria defining centrality. Image 1: In your opinion, is the site of the nodule: central/peripheral? Image 2: In your opinion, is the site of the nodule: central/peripheral? Images 3a and 3b: In your opinion, is the site of the nodule: central/peripheral? Image 4: Which of the following lines best defines centrality? A lines/B lines. Image 5: Which of the following lines best defines centrality? A lines/B lines. Image 6: Which of the two lines demarcates a central tumor? A line (inner third)/B line (inner two thirds). Image 7: Which of the two lines demarcates a central tumor? A line (inner third)/B line (inner two thirds). Image 8: In your opinion, is the site of the nodule: central/peripheral? Image 9: In your opinion, is the site of the nodule: central/peripheral?
- 2)
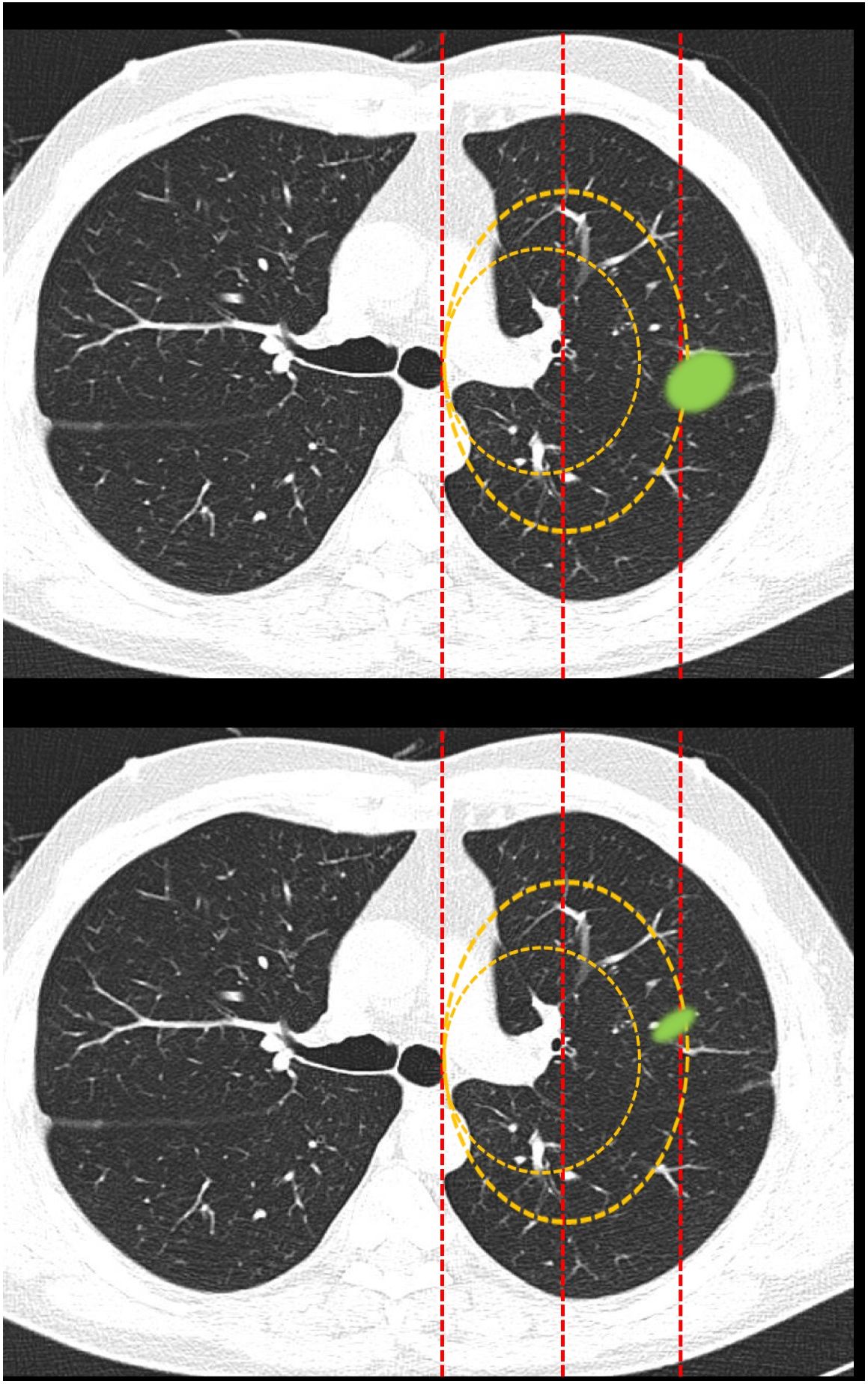
Classification of lesions extending beyond dividing lines (Fig. 2): this block evaluated the uniformity of criteria for classifying a lesion as central [according to a definition of centrality (in the inner two thirds) previously established in the text of the question] if it exceeded beyond dividing lines previously plotted on the image.
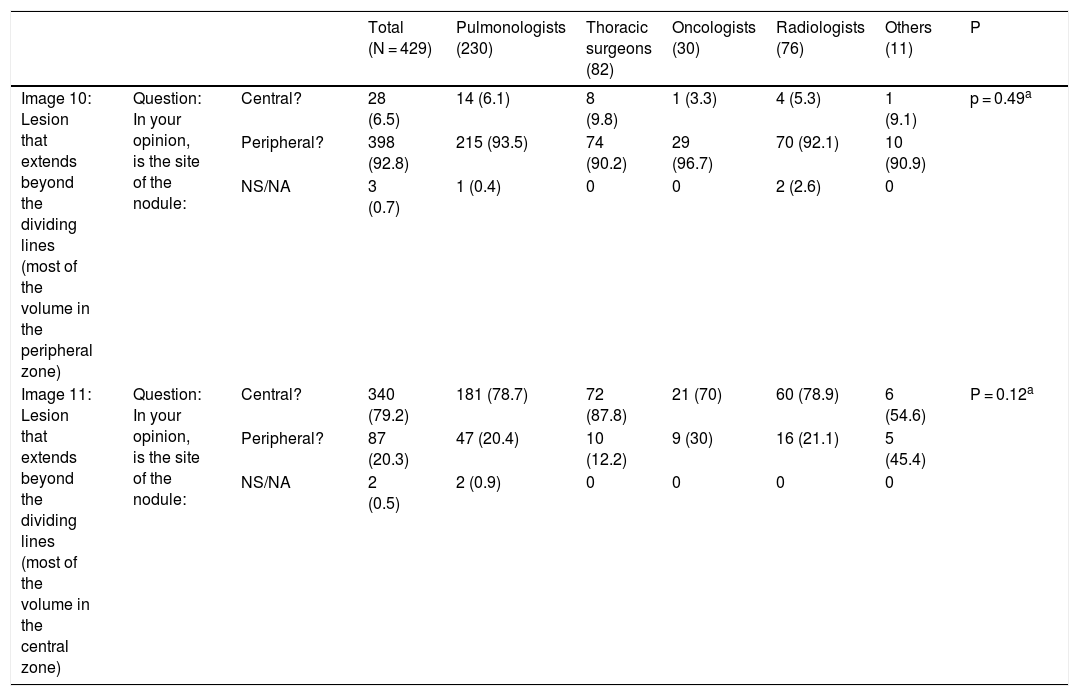
Fig. 2.This block evaluated the uniformity of criteria used to classify a lesion as central in lesions that extend beyond the dividing lines. Image 10: If a tumor located in the inner two thirds of the hemitorax is defined central, in your opinion, is the site of the nodule: central/peripheral? Image 11: If a tumor located in the inner two thirds of the hemitorax is defined as central, in your opinion, is the site of the nodule: central/peripheral?
- 3)
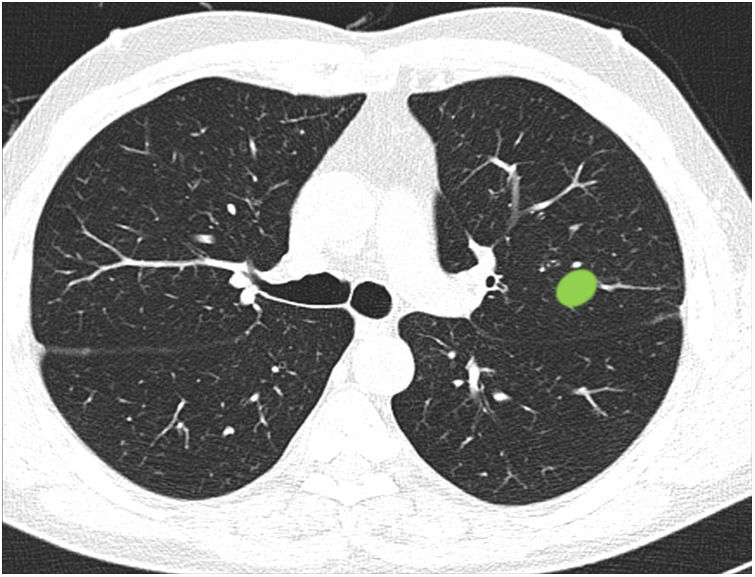
Ability to properly classify a lesion in a site between central and peripheral in the absence of dividing lines (Fig. 3): this block evaluated the uniformity of criteria for classifying a lesion as central (according to a definition of centrality [in the inner two thirds] previously established in the text of the question]) in the absence of dividing lines.
The results of the survey were collected in a database and analyzed using the Stata S/E program (StataCorp. 2014. Stata Statistical Software: Release 13. College Station, TX: StataCorp LLC). Categorical variables were expressed as absolute and relative frequencies and continuous variables as mean (m) and standard deviation (SD) in the case of normal distribution and as median (M) and interquartile range (IQR) (25%–75%) in the case of non-normal distribution. Questionnaire responses were compared between different specialties using Fisher’s exact test. The answers to different questions were also compared using Fisher’s exact test. A result was considered statistically significant in case of p < 0.05.
ResultsA total of 430 respondents completed the questionnaire. The majority were aged 30–45 years (45.9%), were pulmonologists (53.5%), and were directly involved in the diagnosis of lung cancer (86.5%).
Definition of centralityAlmost half of the respondents selected “lesions in contact with hilar structures” (49.7%) as the definition of central tumor location (Table 1 [Question 1]). The lines most often selected to delimit centrality were B lines (concentric lines arising from the pulmonary hilum) (89%) (images 4 and 5). The “peripheral lesion positive control” image (image 1) was classified as peripheral by all respondents while the “central lesion positive control” image (image 2) was classified as central by three-quarters of the respondents. The image selected as “SEPAR central lesion positive control” (images 3a and 3b) was mostly classified as peripheral (71.1%). Most respondents voted to restrict centrality to the inner third of the hemithorax, instead of the inner two thirds for both A lines (image 6) (310 [72.3%]) and B lines (image 7) (339 [79%]). The presence of an endobronchial lesion influenced the classification of a lesion as central, since only 7% of respondents initially classified an image (image 8) as such, yet this proportion increased to 36.1% (image 9) when information from an endobronchial image was added (Fisher’s exact test p < 0.01). Unlike other questions, statistically significant differences between specialties were observed for this image (Fisher's exact test p < 0.01) (Table 1).
Uniformity of definitions of centrality.
| Total (N = 429) Number (%) | Pulmonologists (N = 230) Number (%) | Thoracic surgeons (N = 82) Number (%) | Oncologists (N = 30) Number (%) | Radiologists (N = 76) Number (%) | Other (N = 11) Number (%) | P | |||
|---|---|---|---|---|---|---|---|---|---|
| Image 11: | Question: In your opinion, is the site of the nodule: | Central | 0 | 0 | 0 | 0 | 0 | 0 | |
| Peripheral | 429 (100) | 230 (100) | 82 (100) | 30 (100) | 76 (100) | 11 (100) | |||
| NS/NA | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Image 22: | Question: In your opinion, is the site of the nodule: | Central | 325 (75.8) | 188 (81.7) | 49 (59.7) | 24 (80) | 53 (69.7) | 11 (100) | <0.01a |
| Peripheral | 104 (24.2) | 42 (18.3) | 33 (40.3) | 6 (20) | 23 (30.3) | 0 | |||
| NS/NA | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Image 33: | Question: In your opinion, is the site of the nodule: | Central | 123 (28.7) | 75 (32.6) | 14 (17.1) | 17 (56.7) | 14 (18.4) | 3 (27.3) | <0.01a |
| Peripheral | 305 (71.1) | 154 (70) | 68 (82.9) | 13 (43.3) | 62 (81.6) | 8 (72.7) | |||
| NS/NA | 1 (0.2) | 1 (0.4) | 0 | 0 | 0 | 0 | |||
| Image 44: | Question: Which of the following lines best defines centrality? | A lines | 47 (11) | 23 (10) | 9 (11) | 3 (10) | 11 (14.5) | 1 (9.1) | P = 0.82 |
| B lines | 382 (89) | 207 (90) | 73 (89) | 27 (90) | 65 (85.5) | 10 (90.9) | |||
| NS/NA | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Image 55: | Question: Which of the following lines best defines centrality? | A lines | 39 (9.1) | 19 (8.3) | 6 (7.3) | 4 (13.3) | 9 (11.8) | 1 (9.1) | P = 0.82 |
| B lines | 389 (90.7) | 210 (91.3) | 76 (92.7) | 26 (86.7) | 67 (88.2) | 10 (90.9) | |||
| NS/NA | 1 (0.2) | 1 (0.4) | 0 | 0 | 0 | 0 | |||
| Image 66: | Question: Which of the two lines demarcates a central tumor? | Inner third | 310 (72.3)b | 172 (74.8) | 53 (64.6) | 18 (60) | 58 (76.3) | 9 (81.8) | p<0.03a |
| Inner two thirds | 76 (17.7) | 45 (19.6) | 15 (18.3) | 5 (16.7) | 10 (13.2) | 1 (9.1) | |||
| NS/NA | 43 (10) | 13 (5.6) | 14 (17.1) | 7 (23.3) | 8 (10.5) | 1 (9.1) | |||
| Image 77: | Question: Which of the two lines demarcates a central tumor? | Inner third | 339 (79)b | 173 (75.2) | 66 (80.5) | 25 (83.3) | 66 (86.8) | 9 (81.8) | P = 0.56 |
| Inner two thirds | 88 (20.6) | 56 (24.4) | 15 (18.3) | 5 (16.7) | 10 (13.2) | 2 (18.2) | |||
| NS/NA | 2 (0.4) | 1 (0.4) | 1 (1.2) | 0 | 0 | 0 | |||
| Image 88: | Question: In your opinion, is the site of the nodule: | Central | 30 (7) | 16 (7) | 8 (9.7) | 1 (3.4) | 3 (3.9) | 2 (18.2) | <0.05a |
| Peripheral | 398 (92.8) | 214 (93) | 74 (90.3) | 29 (96.7) | 73 (96.1) | 8 (72.7) | |||
| NS/NA | 1 (0.2) | 0 | 0 | 0 | 0 | 1 (9.1) | |||
| Image 99: | Question: In your opinion, is the site of the nodule: | Central | 155 (36.1)c | 104 (45.2)c | 30 (36.6) | 4 (13.3) | 14 (18.4)c | 3 (27.2) | P = 0.49 |
| Peripheral | 270 (63) | 126 (54.8) | 50 (61) | 26 (86.7) | 60 (79) | 8 (72.8) | |||
| NS/NA | 4 (0.9) | 0 | 2 (2.4) | 0 | 2 (2.6) | 0 | |||
| Question 1: What definition do you use to classify a lesion as central? | Lesions in contact with the hilum | 213 (49.7) | 99 (43) | 55 (67) | 17 (56.7) | 37 (48.7) | 5 (45.4) | <0.03a | |
| Lesions in the inner third | 51 (11.9) | 25 (10.9) | 8 (9.8) | 3 (10) | 13 (17.1) | 2 (18.2) | |||
| Lesions in the inner two thirds | 143 (33.3) | 89 (38.7) | 18 (22) | 8 (26.7) | 25 (32.9) | 3 (27.3) | |||
| Lesions in contact with the mediastinum | 3 (0.7) | 2 (0.9) | 0 | 1 (3.3) | 0 | 0 | |||
| Endobronchial lesions | 11 (2.6) | 11 (4.8) | 0 | 0 | 0 | 0 | |||
| Other | 7 (1.6) | 4 (1.7) | 1 (1.2) | 1 (3.3) | 0 | 1 (9.1) | |||
| NS/NA | 1 (0.2) | 0 | 0 | 0 | 1 (1.3) | 0 | |||
NS/NA: Not sure/not answered.
The nodule image shows a peripheral lesion (“peripheral lesion positive control”) as defined by the ESTS and ACCP, irrespective of the dividing lines to be used (lines parallel to the midline [A lines] or lines concentric to the hilum [B lines]).
The nodule image shows a central lesion (“central lesion positive control”) as defined by the ESTS and ACCP, irrespective of the dividing lines to be used (A lines or B lines).
The nodule image shows a central lesion according to the SEPAR definition of central tumor location (“SEPAR central control”).
The image shows A lines and B lines in an axial CT slice and respondents should choose between the inner third and inner two thirds as the dividing line for classification of lesions.
The image shows B lines in an axial CT slice and respondents should choose between the inner third and inner two thirds as the dividing line for classification of lesions.
Most survey respondents classified lesions based on whether most of their volume was on one side or the other of a dividing line previously traced on the image. Thus, lesions predominantly located on the peripheral side were classified as peripheral (image 10) (92.8%) and those predominantly located on the central side were classified as central (image 11) (79.2%), with no differences between specialties (Table 2).
Classification of lesions that extend beyond dividing lines (most of the volume in the peripheral zone versus most of the volume in the central zone). Most respondents believe that lesions should be classified according to where most of their volume is located. No significant differences were found between different specialty groups for either the first question or the second (aFisher exact test).
| Total (N = 429) | Pulmonologists (230) | Thoracic surgeons (82) | Oncologists (30) | Radiologists (76) | Others (11) | P | |||
|---|---|---|---|---|---|---|---|---|---|
| Image 10: Lesion that extends beyond the dividing lines (most of the volume in the peripheral zone) | Question: In your opinion, is the site of the nodule: | Central? | 28 (6.5) | 14 (6.1) | 8 (9.8) | 1 (3.3) | 4 (5.3) | 1 (9.1) | p = 0.49a |
| Peripheral? | 398 (92.8) | 215 (93.5) | 74 (90.2) | 29 (96.7) | 70 (92.1) | 10 (90.9) | |||
| NS/NA | 3 (0.7) | 1 (0.4) | 0 | 0 | 2 (2.6) | 0 | |||
| Image 11: Lesion that extends beyond the dividing lines (most of the volume in the central zone) | Question: In your opinion, is the site of the nodule: | Central? | 340 (79.2) | 181 (78.7) | 72 (87.8) | 21 (70) | 60 (78.9) | 6 (54.6) | P = 0.12a |
| Peripheral? | 87 (20.3) | 47 (20.4) | 10 (12.2) | 9 (30) | 16 (21.1) | 5 (45.4) | |||
| NS/NA | 2 (0.5) | 2 (0.9) | 0 | 0 | 0 | 0 | |||
NS/NA: Not sure/not answered.
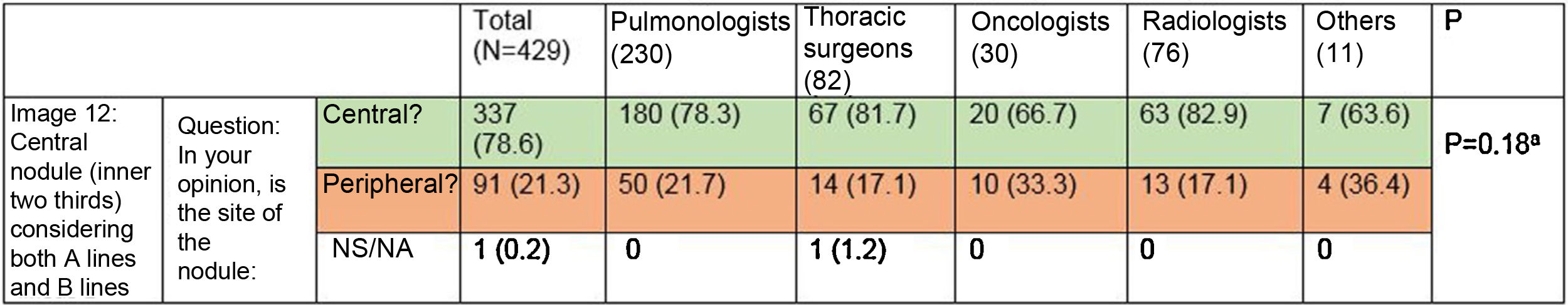
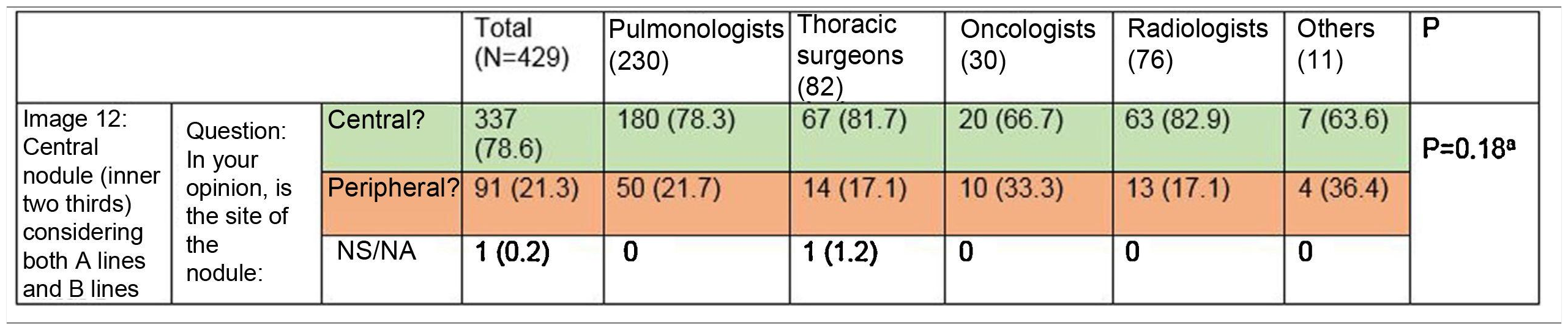
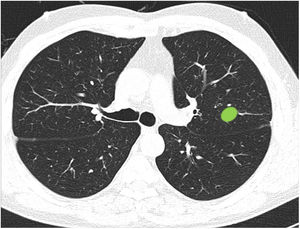
Most respondents (78.6%) were able to classify a central lesion in the absence of dividing lines (image 12), with no significant differences between specialties (Table 3). Similarly, age, sex, or direct involvement in the management of patients with lung cancer did not influence the answer to any of the questions.
Ability to correctly identify a central node in the absence of dividing lines (Figure 12). Most respondents can correctly identify a central lesion (green boxes) in the absence of the dividing lines. No significant differences were observed between groups.
NS/NA: Not sure/not answered.
aFisher’s exact test p = 0.18.
Our survey, conducted among more than 400 professionals involved in the diagnosis and treatment of lung cancer in Spain, shows that the definition of centrality most widely accepted by respondents is not included in any of the clinical guideline proposals. The results also reflect a significant lack of uniformity in the classification of central tumors, although respondents’ responses show distinct patterns: the preferred dividing lines are radial concentric lines arising from the hilum (B lines); the most widely accepted limit for centrality is the inner third; and in most cases it is agreed that a lesion that crosses a dividing line should be classified according to where most of its volume is located. It is also important to note that, despite the subjective nature of the current definitions, most survey respondents were able to properly define a central lesion in the absence of dividing lines.
The concept of centrally located tumors was introduced by Daly et al.7 in 1987. These authors reported that centrally located tumors, which they defined as lesions observed on flexible bronchoscopy or visualized in the inner third of the lung in the anterior-posterior projection of the chest X-ray, were more likely to have occult mediastinal nodal involvement on chest CT. In the 1990s, with the introduction of the first PET machines - with low image resolution - it was accepted that centrally located tumors could hide mediastinal lymphadenopathies located in their vicinity. This phenomenon was known as the hot-spot effect. In the 2000s, additional evidence that centrally located tumors are associated with a higher rate of false negatives in imaging tests has appeared and new definitions of central location have been put forward, such as those proposed by Gómez-Caro et al.4 (lesions in contact with hilar structures [lobar bronchi, main pulmonary or lobar arteries, main pulmonary veins]) or the American Association of Radiation Oncology5 (lesions located less than 2 cm from the bronchial tree). Although the latter was proposed for the detection of patients at risk of toxicity from stereotactic radiation therapy and not for mediastinal staging purposes, these new definitions added to the confusion surrounding the definition of central tumor location. More recently, evidence-based mediastinal staging guidelines have been published by SEPAR, ACCP, and ESTS, but these recommendations differ widely in the definition of central tumor location. In our study, in answer to the question “What definition do you use to classify a lesion as central?” (question 1), nearly 50% of respondents voted for the definition put forward by Gómez-Caro4 (lesions in contact with the hilum) followed by the ESTS definition (lesions in the two inner thirds) and that of the ACCP (lesions in the inner third). However, when directly comparing the definitions of the ESTS and the ACCP (images 6 and 7), most respondents chose the latter by restricting centrality to the inner third of the hemithorax. Very few respondents chose the definition proposed by SEPAR in answer to question 1, and the “SEPAR central tumor positive control” image was mostly classified as peripheral. These results show that respondents deviate significantly from clinical practice guidelines.
In addition to the lack of uniformity, clinical guidelines are very brief when defining centrally located tumors. This brevity leaves several questions arising in routine clinical practice unanswered, such as: What portion of the tumor should be considered for classification (inner border, outer border, center)? Which lines should be used to delimit the thirds of the lung? How should tumors that extend beyond dividing lines be classified? The second question has historically been answered by using vertical dividing lines parallel to the midline (A lines), as defined by Daly et al.7 However, in 2007, other authors reported using concentric lines arising from the hilum (B lines)8 and more recently Shin et al.9 have used concentric curved lines arising from the midline. In our survey, the lines most widely accepted for demarcating the lung thirds were the B lines, which is in line with the most widely accepted definition of central tumor location (in contact with hilar structures), and shows that most respondents associate a central location with the pulmonary hilum rather than the midline. As for lesions that cross the dividing lines, instead of defining them all as central, most respondents classified them according to the location of the greatest part of their volume.
The role of centrally located tumors as a predictor of mediastinal disease has come under scrutiny since the publication of recent studies showing conflicting results.4,8,10–15 Some authors believe that these disparities may be due to the use of different definitions for central tumor location in each of the studies.16 For this reason, 3 studies using varying definitions of central location have been published in the last 2 years.9,17,18 As a result, none of the definitions proposed in the guidelines was associated with occult mediastinal nodal disease in any of these series. Since tumors greater than 3 cm and N1 tumors in imaging tests are themselves associated with occult mediastinal nodal disease, with N2 disease figures of around 11% and 26%, respectively,19,20 it is important to note that a central location is only an indication for invasive mediastinal staging in the case of pulmonary nodules (T1N0 in imaging tests). In this regard, only 3 studies have included a population exclusively composed of patients with T1N0 tumors in imaging tests.18,21,22 In these series, the prevalence of occult mediastinal nodal disease was low (between 4.7% and 8%) and no association was found between occult disease and tumor location. The role of centrally located tumors in mediastinal staging is therefore controversial, firstly, because of the lack of uniform definitions and, secondly, due to doubts about their association with occult mediastinal disease in patients with T1N0 tumors in imaging tests.
Our study features a significantly higher number of respondents than similar studies,6 but our results, nevertheless, must be interpreted with the same caution as any survey and cannot be considered representative of the entire medical community that treats lung cancer patients in Spain. However, we believe that the lack of a uniform definition of centrality and the discrepancy with clinical guidelines is striking and worthy of reflection.
ConclusionsIn summary, this study highlights the lack of uniformity in the definition of central tumor location used by professionals responsible for the management of patients with NSCLC who participated in the survey. It also shows a low adherence by survey respondents to clinical guidelines for certain aspects of central tumor location, mainly in terms of the spatial reference of centrality, determined by the guidelines as the midline yet considered by most survey respondents as the hilum. Despite the limitations of our study, the results reflect the need to include a broader, more uniform definition of centrally located tumors in the next clinical guidelines, if this criterion continues to be specified as an indication for invasive mediastinal staging.
Conflict of interestsThe authors state that they have no conflict of interests.
Please cite this article as: Martínez-Palau M, Trujillo-Reyes JC, Jaen À, Call S, Martínez-Hernández NJ, Provencio M, et al. ¿Cómo clasificamos un tumor central? Resultados de una encuesta multidisciplinaria propuesta desde el área de Oncología Torácica de SEPAR. Arch Bronconeumol. 2021;57:359–365.