Lung histopathology studies in patients who die with COVID-19 have helped us to understand lung damage caused by the SARS-CoV-2 virus.1,2 In the context of the COVID-19 pandemic, autopsy is limited by various factors. The Spanish Society of Pathology (SEAP) recommends that due to the biological risk of contagion to the pathologist performing the procedure and the risk of viral spread, autopsies should be restricted to centers with Biosafety level 3 facilities that are equipped with type II biological safety cabinets and HEPA filters3; however, such resources are uncommon in Spain.3 In view of these limitations, SEAP proposed that in centers where biosafety conditions could not be met, postmortem samples could be collected from patients as an alternative to autopsy.4 In a recent systematic review of lung histopathology studies in COVID-19 infection, 33 of the 171 (19%) samples were postmortem lung biopsies.5 None of the published studies were conducted in hospitals in Spain.
In our hospital, we developed a protocol for obtaining percutaneous pulmonary biopsies in the immediate postmortem period that was approved by the medical research ethics (protocol number: HCB/2020/0487). Consent was obtained from the patient's relatives in all cases. In the case of a patient with a diagnosis of COVID-19, the thoracic surgery team was immediately contacted to obtain core-needle biopsy samples. During the study period, May 4–27, 2020, biopsies were performed on a total of 13 patients. Patient characteristics and treatments are shown in Table 1.
General characteristics of the 13 study patients, n (%)/median (percentiles: 25–75).
| Sex | |
| Women, n (%) | 4 (31) |
| Men, n (%) | 9 (69) |
| Mean age | 78 (68–83) |
| Previous diseases | |
| Respiratory diseasesa, n (%) | 2 (15) |
| Arterial hypertension, n (%) | 7 (54) |
| Diabetes mellitus, n (%) | 4 (31) |
| Cardiovascular disease, n (%) | 3 (23) |
| Treatment | |
| Hydroxychloroquine/azithromycin, n (%) | 11 (84) |
| Lopinavir/ritonavir, n (%) | 9 (69) |
| Tocilizumab, n (%) | 5 (38) |
| Anakinra, n (%) | 6 (46) |
| Corticosteroids, n (%) | 11 (85) |
| Antibiotics, n (%) | 10 (77) |
| Admission to intensive care unit | 9 (62) |
| Orotracheal intubation+mechanical ventilation | 4 (31) |
| Non-invasive mechanical ventilation | 4 (31) |
| High-flow oxygen therapy | 4 (31) |
| Days of hospital admission | 30 (5-44) |
Biopsies were performed by personnel using full personal protective equipment, with manual (Tru-Cut® Biopsy Device 14G×15cm) or semi-automated (Bard® Mission™ Disposable Core Biopsy Instrument 14G×16cm) needles. Specimens were obtained using anatomical landmarks, establishing 3 puncture areas for each hemithorax: anterior, lateral, and posterior. Each area was punctured as often as required to obtain a cylinder of lung tissue. The samples were immediately placed in pre-filled formalin safety capsules (SafeCapsule SC021 and SC022, DiaPath) for histopathological analysis. The samples were fixed in formalin for 24h and then embedded in paraffin blocks, following the standard procedure. In addition to hematoxylin–eosin, Masson's trichrome staining was performed in each case to evaluate interstitial fibrosis, Perls stain to show hemosiderin deposits, and methenamine silver stain to identify fungi. Immunohistochemical staining was performed for smooth muscle actin to identify myofibroblasts.
Table 2 shows the results of the biopsies. Overall, lung tissue samples were obtained from 73% of the procedures (57 out of 78 punctures). No SARS-CoV-2 infections associated with the procedure were reported. The most frequent histopathological pattern was diffuse alveolar damage (DAD) (n=9; 75%). Exudative phase DAD was observed in 2 patients and proliferative phase DAD in 8 (66.7%). In 3 patients, DAD changes were also associated with acute fibrinous organizing pneumonia (AFOP) foci, and in 2 patients changes were associated with foci of organizing pneumonia. Organizing pneumonia changes were identified in 3 patients: 2 associated with DAD, and 1 with alveolar hemorrhage. Moderate or severe interstitial cell enlargement was observed in the vast majority of samples (n=7; 58.3%). In all these cases, myofibroblasts were observed in the interstitium. Intra-alveolar fibrin was observed in 6 cases (50%). The core-needle biopsy also helped reach additional diagnoses (Table 2): pulmonary hemorrhage (n=3), smoking-related pulmonary fibrosis (n=1), Pneumocystis jirovecii infection (n=1), and carcinomatous lymphangitis (n=1). No vascular microthrombi were observed in the specimens analyzed. Samples with the most significant representative radiological and histological findings are included in the supplementary material in Appendix B Fig. 1S–5S).
Histopathological findings.
| Case | Organizing pneumonia | Enlarged interstitial cells | Interstitial myofibroblasts (actin) | Hyaline membranes | Intra-alveolar fibrin | Inflammation | Pneumocyte reactivity | Cytopathic changes | Hemosiderophages | Vascular microthrombi | Histological diagnosis | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | No | Moderate | Yes | No | Yes | Focal PMNs | Yes | Yes | Focal | Not observed | AFOP/DAD-p | |
| 2 | Yes (Focal) | Moderate/severe | Yes | No | No | No | Yes | No | No | Not observed | Focal organizing pneumonia/DAD-p | |
| 3 | No | Moderate | Yes | No | Yes | Focal PMNs | Yes | Yes | No | Not observed | AFOP/DAD-p | |
| 4 | No | Moderate | Yes | No | Yes | No | Yes | Yes | Yes | Not observed | AFOP/DAD-p | Emphysema+SRIF+bleeding |
| 5 | Yes | Mild | No | No | No | No | No | No | Yes | Not observed | Organizing pneumonia | Hemorrhage |
| 6 | No | Very mild | Very scant | No | No | No | No | No | No | Not observed | Minimal interstitial changes | |
| 7 | No | No | No | No | No | No | No | No | No | Not observed | Carcinomatous lymphangitis with minimal parenchymal changes | |
| 8 | No | Severe | Yes | No | No | Chronic | Yes | Yes | No | Not observed | DAD-p | |
| 9 | No | Mild (focal) | Yes (Focal) | Yes | Focal | PMN | Yes | Yes | No | Not observed | DAD-ex with DAD-p foci and pneumonia | |
| 10 | No | Severe | Yes | No | Yes | Chronic | Yes | No | No | Not observed | DAD-pPneumocystis infection | Emphysema |
| 11 | Focal | Mild | Very scant | No | Yes (Focal) | Focal PMNs | Focal | No | No | Not observed | Minimum changes and focal AFOP | |
| 12 | Yes | Mild | Scant | Yes | No | Focal chronic | No | No | No | Not observed | Focal organizing pneumonia/DAD-ex | |
| 13 | No | Severe | Yes | No | Focal | Focal PMNs | Yes | Yes | No | Not observed | DAD-p |
AFOP: acute fibrinous organized pneumonia; DAD: diffuse alveolar damage; DAD-ex: exudative phase; DAD-p: proliferative phase; SRIF: smoking-related interstitial fibrosis (smoking-related pulmonary fibrosis).
This is the first paper in Spain to publish data from percutaneous pulmonary biopsies from COVID-19 pneumonia, showing that it is a safe and effective alternative when autopsy cannot be performed. Regarding histopathology, the most frequent findings were diffuse alveolar damage in any of its phases, patterns of organized pneumonia, and AFOP, in line with other studies.5 The lung biopsies also confirmed other clinical-radiological complications associated with SARS-CoV-2 infection (Fig. 1).
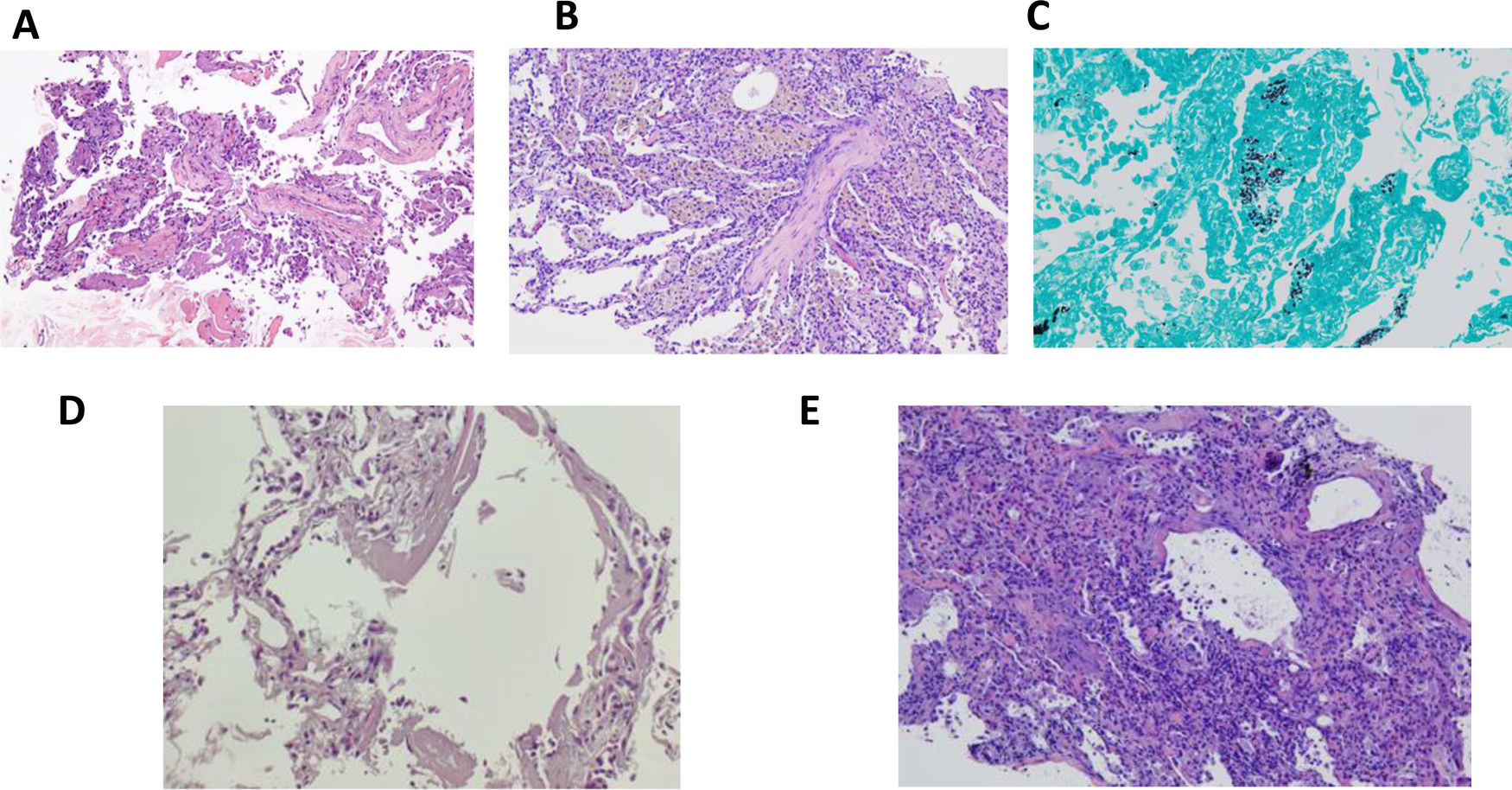
Patient 4: (A) AFOP. Presence of fibrin in alveoli (H&E, ×200). Patient 5: (B) Organizing pneumonia with pseudo-polyp of connective tissue in the center, and the presence of pigmented macrophages in adjacent alveoli, corresponding to hemosiderophages. No pneumocyte reactivity (H&E, ×100). Patient 9: (C) Presence of microorganisms with Pneumocystis jirovecii morphology (silver methenamine stain, ×100). Patient 12: (D) Presence of hyaline membranes in alveolar lumens (H&E, ×100). Patient 13: (E) Diffuse alveolar damage in proliferative phase with interstitial thickening showing pneumocyte reactivity (H&E, ×100).
This study is the largest published series of patients with postmortem biopsies. However, it has some limitations. Firstly, it reports a limited number of patients from a single hospital facility. Secondly, in contrast to autopsy, which would be the technique of choice, the specimens obtained reflect only a small area of the lung compared with the information that can be obtained from an autopsy. However, the findings obtained are very similar to the results of published autopsies, underlining the reliability of this technique.5
In conclusion, postmortem core-needle pulmonary biopsy is a safe and effective method for the histopathological study of COVID-19 pneumonia. In the setting of a well-coordinated multidisciplinary team, it may be an alternative in cases in which lung tissue samples are required and autopsy cannot be performed.
FundingThis study was funded by the SLT008/18/00176 grant and by the Department of Health of the Generalitat de Catalunya in the 2019–2021 competitive call for grants for the funding of programs and instrumental actions included in the Strategic Plan for Health Research and Innovation 2016–2020. It also received funding from Fondos FEDER (PI19/01152), SEPAR, SOCAP, FUCAP, and Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS). This study was also funded with ad hoc sponsorship grants for COVID-19 research, proceeding from donations from citizens and organizations to the Hospital Clinic of Barcelona-Clinical Foundation for Biomedical Research (DN040703).
Conflict of interestsThe authors declare that they have no conflict of interests related with the contents of this study.
The authors would like to thank all the healthcare professionals who participated in the study and who have treated patients admitted for COVID-19.