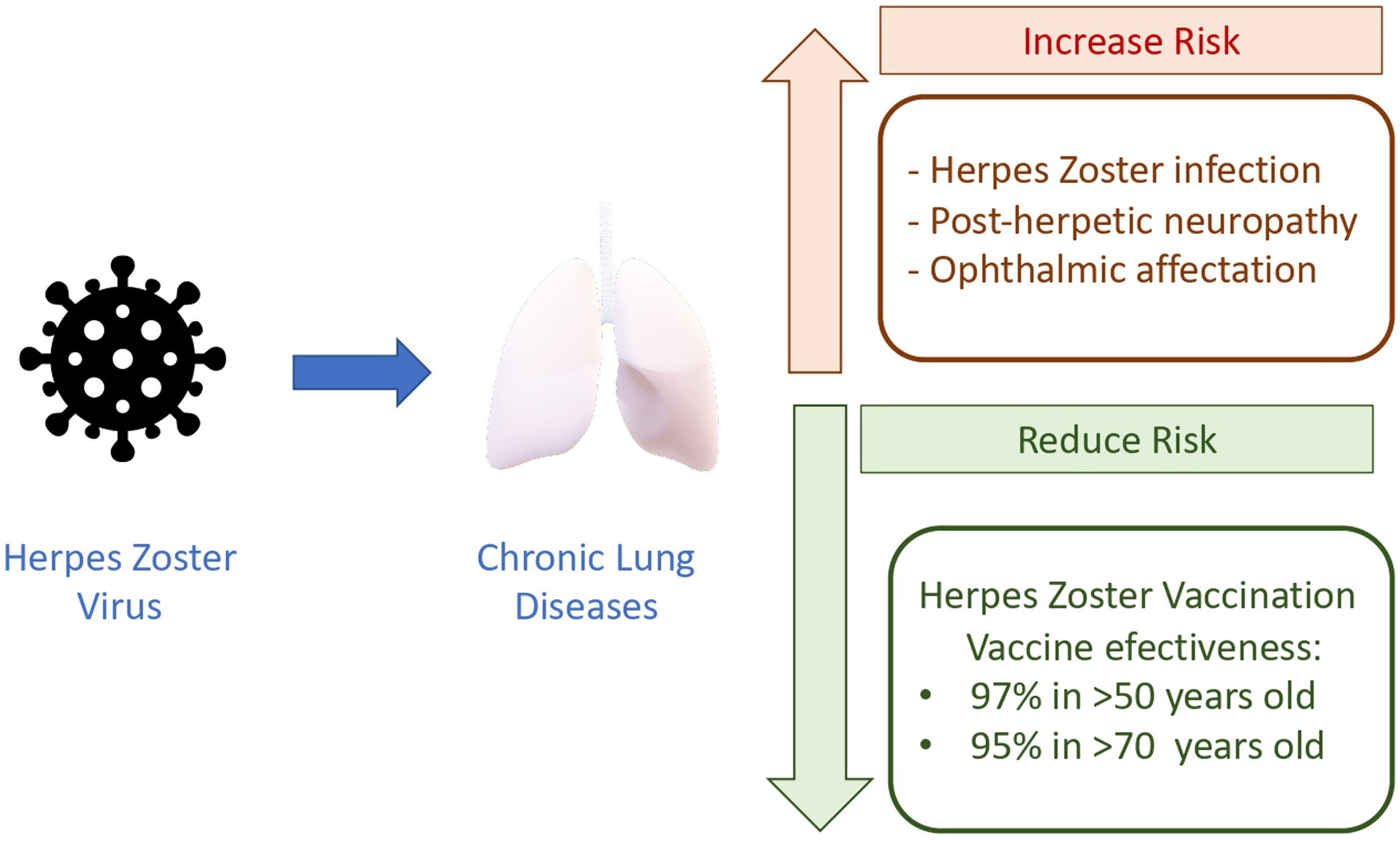
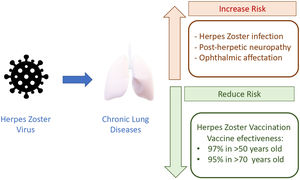
Herpes zoster (HZ) is an infectious disease caused by the reactivation of the latent varicella zoster virus which lies in the spinal ganglia after a primary infection in childhood. It has been estimated that the virus is present in more than 90% of adults, making the potential patients at risk a large amount of population.1 Its reactivation is attributed to changes in cell-mediated immunity that mainly occur due to age and immunosenescence. Therefore, the incidence of HZ increases progressively in adulthood, with an inflection point after 50 years. In Spain, annual incidence is 351 episodes per 100,000 habitants, it increases to 877 in the age group>50 years, and approximately 68.8% of episodes appear after this age.2
Despite the fact that HZ infection is not causing mortality, the burden of disability and costs due to the acute phase, requirement of hospital admission and treatment and, mainly, the chronic complications (post herpetic neuralgia (PHN) and ophthalmic HZ) is enormous. PHN may occur in 5–30% of patients, is more frequent in those aged>50, and its impact remains for months or years. Ophthalmic HZ may appear in approximately 10% of patients, with the potential risk of blindness. Additionally, the burden of HZ infections is expected to rise due to increased life expectancy and the higher survival of patients with debilitating chronic conditions.
Among susceptible patients, those with chronic respiratory diseases, mainly COPD and asthma, are the most frequent and well-studied. Marra F. et al., in a meta-analysis, determine the odds ratio (OR) depending on several comorbid conditions and immunosuppressed conditions (HIV and malignancies).3 Beyond age, COPD patients had an increased risk estimated in OR 1.41 (95% CI, 1.28–1.55), and in asthmatic patients the risk was OR 1.24 (1.16–1.31). In a large retrospective population cohort (2,289,485 persons); 161,317 with COPD and 11,726 with post herpetic neuralgic confirm the increased risk in COPD, with an OR 1.88 (1.77–1.99), showing some differences in risk in relationship with corticosteroid treatment.4,5 The risk is slightly higher in those using inhaled corticosteroids compared to those without (OR 1.61 vs 1.45). In this line, in a cohort study in Taiwan 2004–2006, including a large group (8486 persons with COPD and 33,944 controls), the highest OR reported was found in COPD patients treated with oral corticosteroids (OR 3.00), next in those with inhaled corticosteroids (OR 2.09), and next in those without (OR 1.67).6 As COPD patients present chronic inflammation and impairment of the innate and adaptive immune system, the additional effect of corticosteroid treatment makes sense. However, it must be stated that corticosteroid therapy could be also a surrogate marker of COPD severity. Asthmatic patients exhibit an imbalance in Th1 and Th2 immunity: elevated Th2 and low Th1 immunity. This low Th1 immunity and the impaired innate immune pathways could increase the risk of HZ infection.
The consequences in our patients are that they can develop post-herpetic neuralgia (5–30%), which produces a significant reduction in quality of life for months.7 Noteworthy, our chronic respiratory patients usually have additional comorbid conditions and ≥50 years of age, therefore the probability of adding more risk factors could contribute to a great increase of risk. Sanofova et al. have estimated that the risk of ophthalmic HZ in asthmatic patients increases +90%, and +20% for post-herpetic neuralgia. In COPD the risk for ophthalmic HZ increases +53%.8
The irruption of a new HZ vaccine may modify the clinical scenario and avoid episodes and complications. The new HZ vaccine is composed by a combination of antigen – glycoprotein E – and an adjuvant system – AS01B – to induce a potent and sustained immune response mediated by antibodies (humoral) and memory cells. Cell-mediated immunity, especially CD4 T cells, correlates with protection.9 The advantages of this new vaccine in comparison with a live attenuate vaccine is that is not contraindicated in persons with impaired cellular immunity because HZ virus is not able to replicate. Pivotal studies, randomized, placebo-controlled, phase 3, were performed in 18 countries to evaluate the efficacy and safety of HZ/su in adults (≥50 years of age), stratified according to age group (50–59, 60–69, and ≥70 years). In the two arms, participants received two intramuscular doses of the vaccine or placebo 2 months apart. Studies including 15,411 and 13,900 adults recruited in randomized trials have reported the overall vaccine efficacy against herpes zoster was 97.2% (95% confidence interval [CI], 93.7–99.0; p<0.001). Vaccine efficacy was between 96.6% and 97.9% for all age groups.10,11 These trials demonstrated 97% and 90% efficacy against HZ in adults aged ≥50 and ≥70 years over a median follow-up of 3.1 and 3.7 years, respectively. Mild adverse reactions in HZ vaccine were higher in comparison with placebo, but no differences regarding serious adverse events, potential immune-mediated diseases or mortality were found. Regarding longer duration of efficacy, long-term protection against HZ was demonstrated in an extension of the study until 9.6 years, with a vaccine efficacy of 81.6% (95% CI, 75–86%).12
In those aged≤50 years with asthma or COPD there is limited information on the benefit of HZ vaccination, but their increased risk of developing shingles suggests they may also benefit from inclusion in vaccination programs. In fact, the EMA and CDC expand HZ vaccine in >18 years at increased risk or immunosuppressed.13 Supporting this policy, the most recent GOLD report recommends several vaccines for COPD patients and incorporates HZ vaccination.
Aging and chronic lung diseases make a dangerous combination that put our patients in high risk for infection and its complications, ophthalmic HZ and PHN (Fig. 1). Due to the increased life expectancy, one in three persons will develop HZ infection during lifetime and they are more likely to develop complications. All preventive measures and HZ have already been included in the adult vaccination schedule due to its efficacy. The problem is that there is a very low awareness of the HZ risk in our chronic respiratory patients and, more important, the risk is underestimated among practitioners and specialists. In general, vaccination rates for respiratory infections in adults remain low although it has been demonstrated that infections provoke progression of the disease and decline in life quality. We need to embrace the new approach about healthy aging, preventive measures and moving from a passive to a more proactive attitude.14 The health and life quality of our respiratory patients deserve it.
Conflict of interestsR. Menéndez: advisory board and participation in symposium and/or educational activities for GSK, Pfizer, Gilead and Advanz Pharma.