This editorial, commissioned by the Board of Arch Bronconeumol, focuses on some of the most prominent clinical and pathophysiological concepts related to the hepatopulmonary syndrome (HPS), an enthralling liver-induced lung vascular disorder prevailing in liver cirrhosis (LC). This seems to be timely in the context of an overwhelming period of research focused to all fields of pulmonary vascular disorders.
Year 1966 represents a quantum leap in the understanding of the structural basis of what ultimately would be named HPS 25 years later.1 In that year, Berthelot et al.2 showed that the post-mortem lung findings in patients with LC consisted of pronounced pulmonary vessel dilatations at both the pre-capillary and capillary levels, with or without associated pleural spider naevi in an otherwise intact pulmonary architecture. That study further dug into the intriguing nature of the link between oxygen desaturation and intrapulmonary arterial shunt in the lungs of LC, a problem that remained unsettled until that time. During the 60's and 70's, arterial hypoxaemia (PaO2<80mmHg, at rest while breathing ambient air) in patients with LC had been variously attributed to several mechanisms, such as shifts in the oxyhaemoglobin dissociation curve, intrapulmonary and porto-pulmonary shunts, alveolar-capillary diffusion limitation, and/or ventilation–perfusion (VA/Q) imbalance, none to them sufficiently convincing, in the apparent absence of cardiac or pulmonary disease.
This enticing dilemma was eventually unraveled by a series of investigations using the multiple inert gas elimination technique (MIGET)3 in patients with LC to elucidate the essences of arterial hypoxaemia.4 The patients showed mild systemic and pulmonary vasodilation, increased alveolar–arterial oxygen gradient (AaPO2) (≥15mm Hg) with or without modest hypoxaemia and mild hypocapnia, and normal lung functions tests. The distributions of VA/Q ratios displayed mild abnormalities (i.e., a small proportion of the cardiac output diverted to areas with a low VA/Q ratio), while intrapulmonary shunt was mildly increased or conspicuously absent. Under hypoxic breathing conditions, both pulmonary artery pressure and vascular resistance slightly increased or did not change, whereas VA/Q distributions remained essentially unchanged; by contrast, during breathing 100% oxygen, VA/Q distributions worsened suggesting release (abolition) of hypoxic pulmonary vasoconstriction (HPV). Of note that diffusion limitation to oxygen, as assessed by the MIGET, was not detected. One of the most outstanding findings however was that patients with cutaneous spider naevi, considered to be a relevant sign of angiogenesis, showed greater liver dysfunction and lower systemic and pulmonary vascular resistance, less hypoxic vascular response, more hypoxaemia, and worse VA/Q imbalance than those without spider naevi. Within the next three years four new studies were published in a row in patients with more severe grades of LC and pronounced gas exchange abnormalities,5–8 thus confirming and expanding our seminal findings. Accordingly, the presence of three of the four well-known intrapulmonary factors governing abnormal oxygenation (VA/Q mismatch, increased intrapulmonary shunt, diffusion impairment to oxygen, and hypoventilation) but hypoventilation was compelling in patients with LC, with the predominance of VA/Q inequality induced by an increased pulmonary blood flow enhanced by the absence or impairment of HPV. An increase in the PaO2 to the breathing of 100% oxygen (≥300mm Hg) often occurs in the HPS, a response that may be increased by an elevated cardiac output in the context of the characteristic hyperkinetic circulation of LC. The presence of oxygen diffusion limitation, essentially reflecting a diffusion–perfusion defect, predominates in advanced stages of the HPS, aggravated by a high cardiac output resulting in a shorter transit time of red cells, which are akin to a low diffusing capacity for carbon monoxide (CO) (DLCO) associated with hepatic dysfunction in general and with the HPS in particular. Likewise, DLCO may be reduced because the alveolar–capillary interface is too wide to allow for complete equilibration of CO with haemoglobin. Arterial hypoxaemia may also be reduced by hyperventilation, which increases the levels of alveolar PO2, and high cardiac output, which raises the mixed venous partial pressure of oxygen, other things being equal. The use of the more sensitive oxygen gradient (AaPO2) to make the diagnosis of HPS becomes important because it can increase abnormally before the PaO2 itself becomes abnormally low as the oxygen gradient compensates for the reduced levels of arterial carbon dioxide (CO2) and hyperventilation, both common in LC. Importantly, the severity of HPS is classified according to the levels of PaO2 breathing ambient air. Likewise, both VA/Q and intrapulmonary shunt worsening constitute the principal mechanism of orthodeoxia (i.e., decreased PaO2 from supine to upright) observed in 30% of the patients with the HPS,9 because of a more rigid and fixed pulmonary vascular tone, less liable to proportionately accommodate gravitational blood flow changes to ventilation in dependent alveolar units.
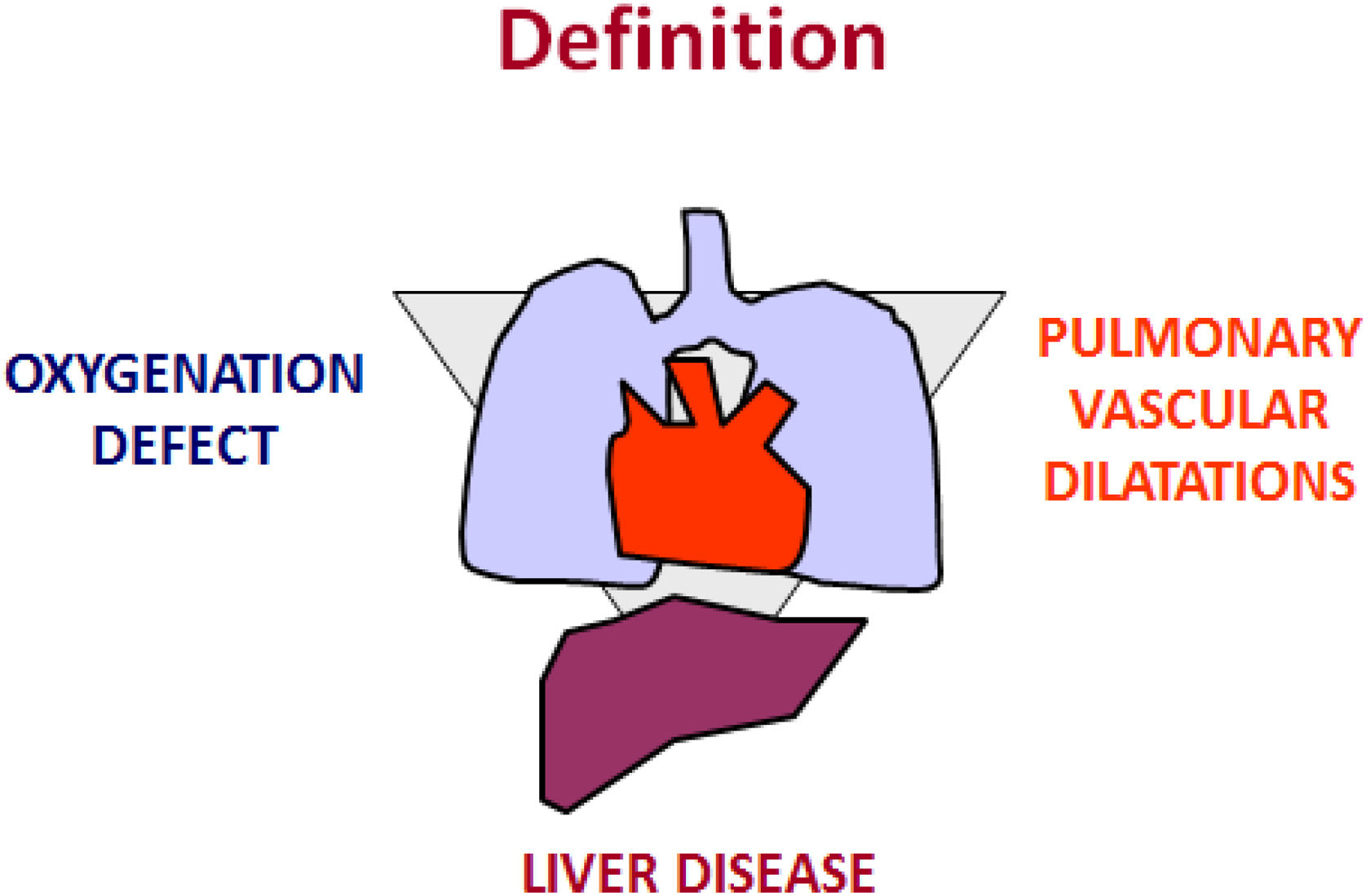
An international task force9,10 defined the term as a clinical triad that encompasses an arterial oxygenation defect, a chronic hepatic disorder (portal hypertension with or without cirrhosis), and the presence of intrapulmonary vascular dilatations (Fig. 1). Data from liver-transplant centres indicate that the prevalence of the HPS ranges from 5 to 32%.10 Advanced liver disease associated with several pulmonary and/or pleural complications is part of the differential diagnosis of the HPS. Clinical judgment may still be necessary, nonetheless, to unravel the severity of arterial hypoxaemia in the HPS and in coexisting commoner pulmonary conditions, such as chronic obstructive airway and interstitial diseases. Patients with HPS are frequently dyspnoeic when not asymptomatic, so the disease can be concealed and its diagnosis delayed. Arterial blood gas measurements and contrast echocardiography are mandatory for the diagnostic work-up.10 The HPS is associated with a profile of abnormal systemic angiogenesis, worse exercise and functional capacity, and an overall increased risk of death.11
Sketch illustrating the three key criteria to establish the diagnosis of HPS: an arterial oxygenation defect (i.e., AaPO2≥15mmHg with or without a PaO2<80mmHg, while breathing ambient air); a chronic hepatic disorder; and the presence of intrapulmonary vascular dilatations (IPVD), as assessed by either positive findings on contrast-enhanced echocardiography with air bubbles or abnormal uptake in the brain [>6%] with radioactive lung-perfusion scanning.
The Rendu–Osler–Weber syndrome (or hereditary haemorrhagic telangiectasia) and post-surgical consequences of cavo-pulmonary anastomoses for various congenital heart conditions can resemble or mimic the HPS.9 Both can be associated with severe arterial hypoxaemia induced by pulmonary vascular dilatation, which may be diffuse or discrete in nature. Krowka and associates have comprehensively investigated a close condition, namely portopulmonary hypertension, sometimes associated with mild hypoxaemia but rarely with severe hypoxaemia, and frequently confused with the HPS.10
Fallon et al.12 have made substantial experimental contributions using the chronic bile duct ligation model in rats and mice and have identified key pathophysiological triggers for the mechanisms that contribute to gas exchange abnormalities in HPS. Relaxation of blood vessels leading to pulmonary vasodilation, angiogenesis,13,14 leading to the development of VA/Q mismatching and increased intrapulmonary shunt, and alveolar dysfunction emerge as the principal mechanisms. The differential function of endothelin (ET) 1 in the pulmonary vasculature results from binding with its receptors: the vascular smooth muscle expressed ET A or B (ETB) receptors mediate vasoconstriction, while the endothelial expressed ETB receptor activates endothelial nitric oxide (NO) synthase and increases NO, ultimately leading to vasodilation.
Regrettably, no effective pharmacological therapies are available as yet so liver transplant has become the only effective therapeutic approach that results in complete resolution of the HPS.15 To wrap up, the HPS remains an enthralling model in Medicine to further pave the pathophysiology and biopathology of pulmonary gas exchange abnormalities in patients with hepatic diseases, a problem that uniquely illustrates the tantalizing interplay between arterial oxygenation and the liver.
Conflict of InterestRR-R has no conflict of interest to disclose.


![Sketch illustrating the three key criteria to establish the diagnosis of HPS: an arterial oxygenation defect (i.e., AaPO2≥15mmHg with or without a PaO2<80mmHg, while breathing ambient air); a chronic hepatic disorder; and the presence of intrapulmonary vascular dilatations (IPVD), as assessed by either positive findings on contrast-enhanced echocardiography with air bubbles or abnormal uptake in the brain [>6%] with radioactive lung-perfusion scanning. Sketch illustrating the three key criteria to establish the diagnosis of HPS: an arterial oxygenation defect (i.e., AaPO2≥15mmHg with or without a PaO2<80mmHg, while breathing ambient air); a chronic hepatic disorder; and the presence of intrapulmonary vascular dilatations (IPVD), as assessed by either positive findings on contrast-enhanced echocardiography with air bubbles or abnormal uptake in the brain [>6%] with radioactive lung-perfusion scanning.](https://static.elsevier.es/multimedia/03002896/0000005900000003/v4_202401230711/S0300289622006706/v4_202401230711/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w98FxLWLw1xoW2PaQDYY7RZU=)







