Vascular endothelial inflammation and enhanced oxidative stress are important factors in the pathogenesis of obstructive sleep apnea syndrome (OSAS). The aim of this study was to determine the levels of heat shock protein (HSP) 27, HSP70, HSP90, l-arginine, and asymmetric dimethylarginine (ADMA) in patients with OSAS and determine their relationship with cardiovascular (CV) risk factors.
Material and methodsForty patients with OSAS, comprising 26 with and 14 without traditional CV risk factors (obesity, hypercholesterolemia, diabetes, hypertension, and smoking), and 20 control subjects without OSAS were included. All patients underwent a full polysomnographic evaluation, and blood samples were obtained in the morning after the night the diagnostic study was performed.
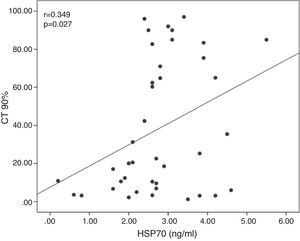
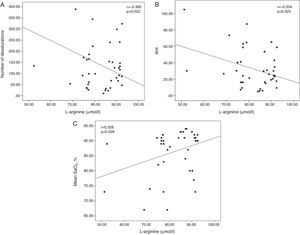
ResultsNo significant differences were found in serum HSP27 and HSP70 levels between the groups. HSP90 and ADMA levels increased significantly, whereas l-arginine levels decreased significantly in patients with OSAS, both with and without CV risk factors, compared with controls, but were not different among the subgroups. In all patients with OSAS, serum HSP70 levels were positively correlated with a percent time with saturation <90% (r=.349, P=.027). Serum l-arginine levels were negatively correlated with desaturation number (r=−.360, P=.022) and apnea-hypopnea index (r=−.354, P=.025) and positively correlated with mean oxygen saturation (r=.328, P=.039).
ConclusionSerum levels of HSP90 and ADMA increased, whereas those of l-arginine decreased in patients with OSAS regardless of CV risk factors. These findings indicate the presence of oxidative stress and endothelial dysfunction in patients with OSAS.
La inflamación del endotelio vascular y el aumento del estrés oxidativo son factores patogénicos importantes del síndrome de apnea obstructiva del sueño (SAOS). El objetivo de este estudio fue determinar las concentraciones de las proteínas de estrés (HSP) 27, HSP70 y HSP90, l-arginina y dimetilarginina simétrica (ADMA) en pacientes con SAOS y establecer su relación con factores de riesgo cardiovascular (CV).
Material y métodosEn el estudio se incluyó a 40 pacientes con SAOS, 26 de los cuales presentaban factores de riesgo CV tradicionales (obesidad, hipercolesterolemia, diabetes, hipertensión y tabaquismo) y 14 no los presentaban, y 20 sujetos de control que no padecían SAOS. A todos los pacientes se les realizó una evaluación polisomnográfica completa y se extrajeron muestras de sangre en la mañana siguiente al estudio diagnóstico.
ResultadosNo se observaron diferencias significativas entre grupos en las concentraciones séricas de HSP27 y HSP70. En los pacientes con SAOS, con o sin factores de riesgo CV, se observaron aumentos significativos de las concentraciones de HSP90 y ADMA y disminuciones significativas de las concentraciones de l-arginina, en comparación con los sujetos de control, aunque no hubo diferencias entre los subgrupos. En todos los pacientes con SAOS, las concentraciones séricas de HSP70 se correlacionaron positivamente con porcentajes de tiempo con saturación <90% (r=0,349; p=0,027). Las concentraciones séricas de l-arginina se correlacionaron negativamente con el número de desaturaciones (r=−0,360; p=0,022) y el índice de apnea-hipopnea (r=−0,354; p=0,025) y positivamente con la saturación de oxígeno media (r=0,328; p=0,039).
ConclusiónLas concentraciones séricas de HSP90 y ADMA aumentaron y las de l-arginina disminuyeron en pacientes con SAOS, independientemente de los factores de riesgo CV que presentasen. Estos resultados indican la presencia de estrés oxidativo y disfunción endotelial en pacientes con SAOS.
Obstructive sleep apnea syndrome (OSAS) is a common health problem caused by repeated episodes of upper airway collapse during sleep. OSAS episodes lead to brain arousal, intrathoracic pressure changes and intermittent episodes of hypoxemia and reoxygenation.1
Heat shock proteins (HSP) are stress proteins that are highly conserved and present in various organisms, from bacteria to mammals. They are produced in response to diverse insults, such as high temperatures, inflammation, ischemia, toxins, smoking, oxidative stress and hypoxia.2 Relatively high concentrations of HSPs released by stressed cells into the extracellular space are found at sites of inflammation.3 As molecular chaperones, intracellular HSPs have a powerful protective effect. However, extracellular HSPs act within the immune system as signal molecules, and modulate the secretion of pro-inflammatory cytokines.4
l-arginine is an essential amino acid required for nitric oxide (NO) production by endothelial nitric oxide synthetase (NOS). Administration of l-arginine improves endothelial function in both animal models and humans with hypercholesterolemia and atherosclerosis.5 Endothelium-derived NO is an important mediator that regulates vasomotor tone. It participates in a wide range of regulatory mechanisms, including inhibition of platelet adhesion and aggregation, inhibition of monocyte adhesion and smooth muscle cell proliferation. Thus, NO plays a fundamental role in vascular homeostasis.6 Asymmetric dimethylarginine (ADMA) is a potent competitive inhibitor of NOS, derived from the catabolism of proteins containing methylated arginine residues. It is excreted by the kidneys or metabolized by dimethylarginine dimethylaminohydrolase, which is inhibited by homocysteine.7 Plasma ADMA levels are elevated in patients with hypercholesterolemia, hypertension and hyperglycemia, and in subjects exposed to tobacco.8
OSAS significantly increases the risk of cardiovascular (CV) diseases such as hypertension, heart failure, arrhythmias and coronary artery disease.9 Vascular endothelial inflammation and enhanced oxidative stress are the starting point for explaining the mechanisms that mediate the known association between OSAS and CV diseases. Repeated episodes of hypoxia and reoxygenation stimulate the production of inflammatory mediators in patients with OSAS. The repeated arousals typical of OSAS and resulting chronic sleep fragmentation can exacerbate the adverse effects of hypoxia/reoxygenation on the endothelial function of patients with OSAS.10
The aim of this study was to determine HSP (HSP27, HSP70 and HSP90), l-arginine and ADMA concentrations in patients with OSAS, to determine whether these markers are associated with CV risk factors, and to investigate their relationship with polysomnographic data.
Materials and MethodsStudy SubjectsWe evaluated 40 patients with OSAS, 26 with and 14 without traditional CV risk factors (obesity [body mass index (BMI)≥30kg/m2], hypercholesterolemia [total cholesterol≥220mg/dl or LDL cholesterol≥140mg/dl], diabetes, hypertension and smoking).11
All patients reported snoring, witnessed apneas, daytime sleepiness, and frequent nocturnal awakenings. The reference group included 20 non-obese, non-smoking control subjects who were symptomatic but with no signs of OSAS in the one-night polysomnography. The quality of sleep and excessive daytime sleepiness were evaluated using the Pittsburgh Sleep Quality Index (PSQI) and the Epworth Sleepiness Scale (ESS). All sleep studies were conducted in the Hospital Universitario Firat Sleep Disorders Laboratory between October 2013 and May 2014.
Polysomnographic RecordingsAll patients attended the laboratory for a full one-night polysomnography using a 44-channel recording system (Compumedics E series, Melbourne, Australia). The following parameters were monitored: brain activity (electroencephalogram with electrodes placed on C3A2, C4A1, O1A2, O2A1, F3A2 and F4A1), muscle tone (electromyogram of chin and both legs), eye movements (electrooculogram), heart rate (electrocardiogram), oxygen saturation (SaO2; digital pulse oximetry), chest and abdominal wall movements (thoracoabdominal belts), airflow (thermistor and nasal cannula pressure transducer) and snoring (microphone).
Polysomnography ScoringThe recordings were scored according to American Academy of Sleep Medicine standardized criteria.12 Apnea was defined as a drop in peak thermal sensor excursion by ≥90% of baseline for ≥10s. Hypopnea was defined as drop in the nasal pressure signal by ≥30% of baseline for ≥10s, causing a ≥3% decrease in the SaO2 pre-event baseline or an arousal. Desaturation was defined as a 3% reduction in the SaO2 compared to baseline. The mean SaO2 was defined as the mean SaO2 recorded during the night. The minimum SaO2 was defined as the lowest SaO2 value recorded during the night. The apnea-hypopnea index (AHI) was defined as the mean number of apneic and hypopneic episodes per hour of sleep. An AHI>5/h was considered diagnostic of OSAS. Subjects with an AHI≤5/h were assigned to the control group.
None of the participants had chronic illnesses (chronic obstructive pulmonary disease, liver cirrhosis, thyroid dysfunction, rheumatoid arthritis, chronic renal failure or psychiatric illnesses) or were taking medication. Patient rights were protected and informed consent was obtained from all participants in compliance with the Declaration of Helsinki. The hospital's Ethics Committee approved the study protocol.
Blood ExtractionsBlood samples were drawn from a peripheral vein the morning after the sleep study. Venous blood was centrifuged at 2000rpm and serum samples were stored at −80°C until analysis.
Determination of Heat Shock Protein and Asymmetric Dimethylarginine ConcentrationsSerum concentrations of HSP27 (Assaypro LLC, St. Charles, MO, USA; catalog no.: EH5001-1), HSP70 (USCN Life, Hubei, China; catalog no.: E0873h) and HSP90 (USCN Life, catalog no.: E0863h) were determined using commercial enzyme-linked immunosorbent assay kits, according to the manufacturer's instructions. Values were recorded in ng/ml. Serum l-arginine and ADMA concentrations were determined using a high performance liquid chromatography (HPLC) kit (catalog no.: Z58010; EUREKA srl-Lab Division, Chiaravalle, Italy), in accordance with the study procedures. The l-arginine and ADMA were separated with the aid of a fluorescence monitor. The fluorescence detector wavelengths for excitation and emission were set at 420nm and 483nm, respectively. The sensitivity for detection of ADMA was <0.05μmol/l, and the linearity was >16μmol/l.
Statistical AnalysisThe statistics program IBM Statistical Product and Service Solutions, version 21.0 was used (IBM SPSS Statistics 21 program, Armonk, NY, USA). The following were used for the analysis, as applicable: Mann–Whitney U test, Kruskal–Wallis analysis of variance and Spearman's and Pearson's correlation analyses. Results are expressed as median±standard deviation. Multiple linear regression models were used for the multivariate analysis, with the HSP90, l-arginine and ADMA values as dependent variables, and age, BMI, AHI, number of desaturations and percentage time with saturation <90% (CT 90%) as independent variables. P values <.05 were considered significant.
ResultsWe evaluated 26 patients with OSAS and CV risk factors, 14 with OSAS but no CV risk factors, and 20 control subjects. Of the subjects with OSAS and risk factors, 19 had more than one risk factor, 2 had arterial hypertension only, 4 were obese, and 1 was diabetic.
The demographic characteristics and baseline polysomnography results of OSAS patients and control subjects are shown in Table 1. By definition, the sleep parameters were abnormal in OSAS patients and normal in controls. BMI, AHI, arousal index, scores on the ESS and PSQI, mean/minimum SaO2, number of desaturations, CT 90% and stage 3 sleep time (%) differed significantly between OSAS patients and controls. No differences were observed between OSAS patients and controls in terms of sex, age or total cholesterol. LDL cholesterol values were significantly higher in patients with CV risk factors than in healthy subjects and patients with no CV risk factors.
Demographic and Anthropometric Data and Polysomnographic Results of Obstructive Sleep Apnea Syndrome (OSAS) Patients and Controls.
| Total OSAS (n=40) | OSAS With CV Risk Factors (n=26) | OSAS Without CV Risk Factors (n=14) | Control Subjects (n=20) | |
|---|---|---|---|---|
| Age, years | 53.4±11.5 | 57.5±10.8b,*** | 45.9±8.8 | 49.3±10.9 |
| Sex, male/female | 29/11 | 17/9 | 12/2 | 14/6 |
| BMI, kg/m2 | 34.9±8.5* | 38.5±8.6a,* | 28.5±1.6**** | 26.7±2.3 |
| ESS | 10.2±4.7*** | 11.3±4.7** | 8.1±4.1 | 6.8±4.1 |
| PSQI | 12.2±4.1* | 12.8±4.1* | 10.9±3.8** | 7.3±1.7 |
| Episodes of AHI/h−1 | 32.5±23.7* | 34.8±23.3* | 28.3±24.6* | 1.9±1.2 |
| Mean SaO2, % | 86.4±7.7* | 83.9±8.4b,* | 91.1±1.9** | 93.0±1.3 |
| Minimum SaO2, % | 73.8±13.5* | 69.2±14.3b,* | 82.5±5.1* | 88.4±2.2 |
| Number of desaturations | 140.7±131.4* | 159.7±147.4* | 105.4±89.4* | 6.4±5.4 |
| CT 90% | 38.3±34.7* | 51.2±35.8b,* | 14.3±13.9* | 2.5±3.8 |
| Arousal index | 15.5±10.1* | 17.3±10.8* | 12.2±7.9*** | 5.4±4.7 |
| REM sleep, % | 13.4±7.9 | 13.7±7.6 | 13.0±8.8 | 16.7±5.9 |
| Stage 3 sleep, % | 18.8±10.1* | 18.1±9.9* | 20.0±10.8** | 32.3±10.2 |
| Total cholesterol, mg/dl−1 | 202.6±31.9**** | 211.9±32.3c,** | 185.5±23.9 | 191.0±11.2 |
| LDL cholesterol, mg/dl−1 | 126.7±28.4** | 135.8±29.3b,** | 109.8±17.1 | 111.4±10.5 |
AHI, apnea-hypopnea index; BMI, body mass index; CT 90%, time with SaO2<90%; ESS, Epworth sleepiness scale; LDL cholesterol, low density lipoprotein cholesterol; mean SaO2, mean oxygen saturation; minimum SaO2, minimum oxygen saturation; PSQI, Pittsburgh sleep quality index.
No significant differences in serum HSP27 or HSP70 concentrations were observed between the 3 groups. Serum HSP90 concentrations were significantly higher in patients with OSAS, with or without risk factors, compared to healthy subjects (P<.001; P=.001). In contrast, these values did not differ significantly between the 2 OSAS patient groups. With respect to ADMA, a similar pattern was observed, as serum ADMA concentrations in OSAS patients with or without CV risk factors were similar and significantly higher than those detected in control subjects (P=.002; P=.005). Serum l-arginine concentrations were significantly lower in OSAS patients, with or without CV risk factors, than in controls (P<.001; P=.003). However, no differences were observed in serum l-arginine concentrations between OSAS patients, with or without CV risk factors, and controls. Table 2 shows the mean±standard deviation of the serum HSP, ADMA and l-arginine concentrations for the 3 subject groups.
Mean Heat Shock Protein, l-Arginine and Asymmetric Dimethylarginine Concentrations in Obstructive Sleep Apnea Syndrome (OSAS) Patients, With and Without Cardiovascular (CV) Risk Factors, and Controls.
| OSAS With CV Risk Factors (n=24) | OSAS Without CV Risk Factors (n=16) | Control Subjects (n=20) | |
|---|---|---|---|
| HSP27 (ng/ml) | 8.06±2.34 | 8.49±1.51 | 7.15±2.59 |
| HSP70 (ng/ml) | 2.80±0.85 | 2.67±1.47 | 2.88±1.47 |
| HSP90 (ng/ml) | 16.72±7.92a | 16.83±7.75b | 8.72±3.39 |
| l-Arginine (μmol/l) | 81.83±9.47a | 82.96±10.74b | 91.62±5.60 |
| ADMA (μmol/l) | 0.64±0.12b | 0.67±0.13c | 0.47±0.19 |
Serum HSP70 concentrations were positively correlated with the CT 90% (r=0.349; P=.027; Fig. 1). Serum l-arginine concentrations were negatively correlated with the number of desaturations (r=−0.360; P=.022) and the AHI (r=−0.354; P=.025), and showed a positive correlation with the mean SaO2 (r=0.328; P=.039; Fig. 2). No significant correlations were observed between HSP, ADMA and l-arginine concentrations.
In the multiple linear regression analysis in which HSP90 was the dependent variable, and age, BMI, AHI, number of desaturations and CT 90% were the independent variables, number of desaturations (β=0.341; P=.034) was an independent predictive factor of HSP90 (R2=0.183); no other relationships were observed with respect to these variables.
DiscussionOur results show that, regardless of CV risk factors, patients with OSAS have more oxidative stress (higher HSP90 concentrations) and endothelial dysfunction (decrease in l-arginine concentrations and increase in ADMA concentrations). These markers indicate vascular endothelial dysfunction and oxidative stress in the early stages of OSAS in patients without traditional CV risk factors, and therefore could be useful in clinical practice.
Circulating concentrations of inflammatory mediators are increased in patients with OSAS, irrespective of the presence of comorbid diseases, such as obesity, which are often associated with this syndrome. The magnitude of the systemic inflammatory response seems to be related with the severity of the OSAS based on AHI.13 The repeated episodes of hypoxia/reoxygenation experienced by patients with OSAS during this transient cessation of breathing lead to systemic oxidative stress and inflammation.10 Exposure to stressors, such as high temperatures, inflammation, ischemia, toxins, tobacco smoke, oxidative stress and hypoxia, trigger the production of HSPs,2 and serum levels of these proteins are elevated in patients with various inflammatory diseases, such as chronic obstructive pulmonary disease, acute coronary syndrome and chronic allograft nephropathy.14–16 Previous experimental studies have shown that production of HSPs increases in response to anoxia, and the increase in HSPs is thought to stabilize and protect the structure and function of proteins.17,18 Anoxia-sensitive models, which show a greater response to heat shock during low oxygenation states, confirm the protective role of HSPs against the damage caused by this stress.19,20
Few studies to date have demonstrated a relationship between HSPs and OSAS. Noguchi et al.21 reported a greater decrease in the levels of basal HSP72 expression during sleep in peripheral blood mononuclear cells of OSAS patients, compared with levels of expression in control subjects before sleep. In another study, HSP70 concentrations were higher in OSAS patients than in control subjects. In this study, HSP70 concentrations rose as the severity of the disease increased, and were significantly and positively correlated with the AHI and oxygen desaturation index. It has also been observed that HSP70 concentrations in non-obese patients with OSAS are higher than those in non-obese control subjects.22 Lavie et al.23 observed that basal HSP70 secretion in monocytes was 1.8 times higher in patients with OSAS, compared to control subjects. They also observed a significant positive correlation between HSP70 and the AHI, CT 90% and systemic markers of oxidative stress. Similarly, the heat stress-induced HSP70 concentrations in patients with OSAS were very low, and a negative correlation was observed between the heat stress-induced HSP70 concentrations and the AHI, CT 90% and oxygen desaturation index. In our study, no differences were observed in HSP27 or HSP70 concentrations in patients with OSAS and control subjects; HSP90 concentrations were significantly higher in OSAS patients, with or without CV risk factors, compared to controls. In the multiple linear regression analysis, the number of desaturations was an independent predictive factor of HSP90; furthermore, serum HSP70 concentrations showed a positive correlation with the CT 90%. These results suggest that in OSAS patients, HSPs are triggered by nocturnal hypoxia. Although patients with or without traditional CV risk factors show lower mean SaO2 levels and higher CT 90% levels, the differences in HSP concentrations between the 2 groups were not significant. Our results suggest that HSP90 concentrations are related with the frequency of the saturations and not with the saturation levels.
Oxidative stress and inflammatory processes, as well as increased leukocyte adhesion due to expression of adhesion molecules, lead to endothelial damage and dysfunction. Consequently, OSAS patients typically have impaired endothelium-dependent vasodilation, which can be partially reversed with continuous positive airway pressure (CPAP) therapy. This suggests the existence of a crucial pathophysiological link between the endothelial dysfunction and intermittent hypoxemia in these patients.24 NO is synthesized in endothelial cells from the conversion of l-arginine to l-citrulline, through the tightly regulated activity of endogenous NOS.25 ADMA is a new risk factor for the development of endothelial dysfunction and CV disease, being an endogenous competitive inhibitor of NOS.26 Chronic elevation of blood ADMA concentrations can contribute to progression of the vascular disease through endothelial damage. This effect appears to involve somewhat more than the reduced availability of NO secondary to the inhibition of NOS.27 Similarly, ADMA may also promote the dissociation of endogenous NOS, which could contribute directly to increasing oxidative stress.28
High ADMA concentrations are associated with various diseases, such as coronary artery disease, peripheral artery disease, hypercholesterolemia, hypertension and renal failure.29–33 Results from the multicenter CARDIAC study suggest that a high ADMA concentration is an independent risk factor for coronary heart disease.29
Some studies have quantified ADMA concentrations in OSAS patients. Ohike et al.34 showed that plasma ADMA concentrations in patients with OSAS decreased when flow-mediated vasodilation improved after CPAP therapy, but the difference between the ADMA concentrations before and after treatment was not significant. Another study suggested that serum ADMA concentrations in patients with overlap syndrome and OSAS are lower after CPAP treatment.35 No differences were detected in ADMA concentrations in patients with OSAS and control subjects in a study that evaluated the diurnal variation between endothelial dysfunction markers and hemostatic factors in patients with OSAS, although the results suggest a significant relationship between the ADMA concentration and arousal index.36 Similarly, Ozkan et al.37 did not observe differences in plasma ADMA concentrations in OSAS patients and control subjects. Another study evaluated soluble CD40 and ADMA concentrations: plasma ADMA concentrations were significantly higher in the OSAS group, irrespective of CV risk factors, compared with controls; this study also found a significant relationship between plasma CD40 and ADMA concentrations.38 Yüksel et al. evaluated the activity of arginase and NO concentrations in patients with OSAS, and observed that arginase activity was high in patients with OSAS, while NO concentrations were low compared with the control subjects. Similarly, the arginase activity was lower in patients with CV risk factors than in those with no risk factors.39
We observed significantly higher serum ADMA concentrations and significantly lower l-arginine concentrations in the group with OSAS, with or without CV risk factors, compared with controls. The incidence of CV disease increases in patients with OSAS, as does the mortality rate due to the disease. It is not surprising that endothelial functions (elevated ADMA and low l-arginine concentrations) are affected in patients with OSAS and traditional CV risk factors. Nevertheless, alterations in these markers in OSAS patients with no traditional CV risk factors could be due to developing endothelial dysfunction due to repeated episodes of apnea, hypoxia or arousals in the absence of these risk factors. The nocturnal hypoxia episodes in patients with OSAS could have decreased l-arginine concentrations and increased ADMA concentrations. However, our study did not find a linear relationship with this increase. Other factors not analyzed in our study could have affected the ADMA and l-arginine concentrations. Previous studies have shown high arginase activity,39 and increased arginase activity as a result of the nocturnal hypoxia could have affected the decrease in the l-arginine concentration in patients with OSAS.
One possible limitation of our study is that serum HSP, l-arginine and ADMA concentrations were only determined before CPAP treatment. Only traditional risk factors were defined, and other risk factors, such as family history, physical inactivity, unhealthy diet, stress, alcohol abuse and passive smoking were not included. Some cases of OSAS could have presented other CV risk factors that we did not consider, and which could have affected our findings.
In conclusion, we observed high HSP90 and ADMA concentrations and significantly low l-arginine concentrations in OSAS patients, regardless of the traditional CV risk factors present. These findings could indicate oxidative stress and endothelial dysfunction in patients with OSAS, even in the absence of traditional CV risk factors. Serum ADMA could be a practical biomarker for the early diagnosis of vascular endothelial dysfunction and atherosclerosis in patients with OSAS.
Conflict of InterestsThe authors declare that they have no conflict of interests.
Please cite this article as: İn E, Özdemir C, Kaman D, Sökücü SN. Concentraciones de proteínas de estrés térmico, l-arginina y dimetilarginina asimétrica en pacientes con síndrome de apnea obstructiva del sueño. Arch Bronconeumol. 2015;51:544–550.