Although not formally considered a clinical practice guideline, the Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease (GOLD) documents have led the recommendations for the management of COPD in the world since 2001. The 2019 version1 has been a significant change in the disease management strategy with some key points which represent both a novelty in disease management and also current challenges that should focus future research. Specifically, one of the major changes has been the differentiation between initial and follow-up pharmacological treatments.
Firstly, GOLD 2019 is strongly based on eosinophil blood count. Interestingly, although the evidence is consistent showing a better response to inhaled corticosteroids (ICS) with increased blood eosinophils, we must keep in mind that all this evidence comes from post hoc, secondary pre-specified, and data modelling analyses.2 Therefore, although it is possible that blood eosinophil counts may help clinicians estimate the likelihood of a beneficial preventive response to ICS, in daily clinical practice it should probably not be considered as a standalone decision-making parameter. In fact, in a population of COPD patients not overlapping with asthma, the blood eosinophil count and percent has not been found to be associated with lung function changes, quality of life, exacerbation frequency, or response to ICS.3 Accordingly, GOLD 2019 states that there is insufficient evidence to recommend that blood eosinophils should be used to predict future exacerbation risk on an individual basis in COPD patients. Rather, different approaches have been proposed to wisely use blood eosinophils count for treatment selection including the clinical context,4 the persistence over time5 or the combination with exhaled biomarkers.6 Altogether, we understand that the message behind including eosinophil blood count is to identify ICS responders (Fig. 1), which is probably a more complex debate that should focus future research. More importantly, the predictor value of blood eosinophils to identify ICS responders will help determine whether this is a strong biomarker or just simply another bystander of COPD.7 This ICS responder might as well match with the concept of asthma-COPD overlap as defined by the Spanish guideline (GesePOC).8,9
Secondly, GOLD 2019 considers the de-escalation of ICS if pneumonia, inappropriate original indication or the lack of response to ICS. However, beyond the debate on the difficulties in defining some of these concepts, the possibility of de-escalation in patients with a well-controlled disease in terms of exacerbations or symptoms is not specifically considered in the document. The decrease in the number of exacerbations for a prolonged time poses a clinical scenario that challenges the necessity of continuing with ICS, even if correctly prescribed initially. Different studies have consistently shown that it is safe to withdraw ICS in patients without previous exacerbations10,11 and recent studies start to point out that ICS prescription could potentially be temporally intensified in determined clinical contexts.12 Therefore, the discussion whether, in a patient receiving ICS, not having exacerbation is due to the effect of the ICS or because of the natural expression of the disease needs further scrutiny. On the other hand, the possibility of de-scalation of double bronchodilator therapy to single bronchodilation in patients with symptoms control has not been explored in the literature and should be evaluated in the future.
Thirdly, switching medication between drugs of the same family represents another interesting debate. Although the GOLD 2019 document suggest considering switching inhaled device or molecules if symptoms are not well-controlled with two long-acting bronchodilators, this idea could potentially be considered in every step of the scalation process. Beyond the potential improvement in the management of a new inhaler after the switch, there is a rationale behind on the specific therapeutic response in a particular patient. Different cross-over studies have clearly showed that different patients respond differently to the same drug.13,14 Therefore, besides the classical statement that the inhaler device technique and treatment adherence should be guaranteed before escalating, future research should focus on the individual response to inhaled drugs even within the same family.
Finally, clinicians should keep in mind that COPD patients do suffer from many other comorbidities which determine the disease presentation and the therapeutic response. Therefore, the potential impact of comorbidities should always be kept in mind in an escalation strategy to improve symptoms or prevent exacerbations. GesEPOC identifies the frequent exacerbator as one relevant clinical phenotype.8 Interestingly, there are a number of comorbid conditions that may impact on exacerbation risk (e.g. cardiovascular disease, gastro-esophageal reflux, bronchiectasis, or vitamin D deficiency), and none of these are treated with ICS.15 Therefore, future research should focus on exploring an algorithm to systematically study COPD patients with persistent exacerbations with the final aim to provide the best approach for each case.
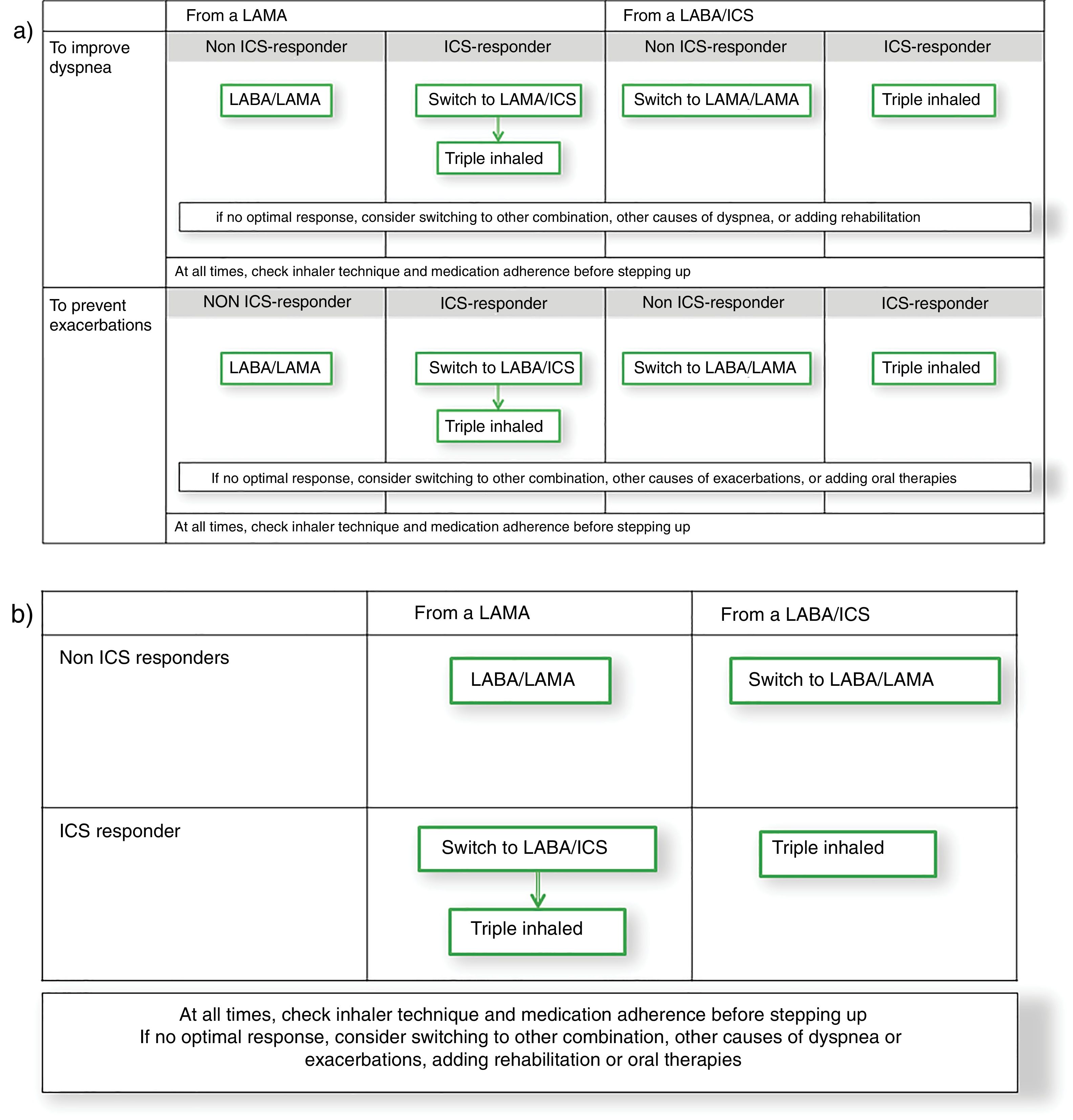
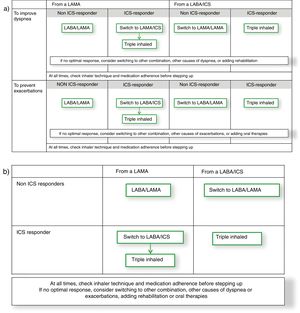
With these debates in mind, an alternative comprehensive scheme combining GOLD 2019 strategy with GesEPOC approach could be built from the GOLD 2019 follow-up treatment algorithm (Fig. 1a). This figure starts from the premise that a patient might initiate therapy with a long-acting muscarinic antagonist (LAMA) or a combination of a long-acting β2 agonist (LABA) with an ICS, as suggested by both GOLD 2019 and GesEPOC.1,8 Thereof, the escalation process would be based on phenotypic clinical presentation with the symptomatic, frequent exacerbator and ICS-responder patient as drivers of the change, as GesEPOC recommends. Interestingly and although the evidence is not as strong for both endpoints, when this approach is built, the recommended escalation strategy is the same for symptoms improvement and preventing exacerbations. Therefore, this diagram could be simplified in a 2×2 table combining the initial treatment (either a LAMA or a LABA/ICS) and being ICS responder or not (Fig. 1b). Consequently, it seems that the real challenge for research is to identify the ICS responder rather than the specific clinical endpoint to improve. Different initiatives have been discussed in the literature to identify these patients, including bronchial reversibility, the blood eosinophil count, the co-expression with asthma, bronchial hyperresponsiveness, or Th2 biomarkers, to name a few examples. Here, GOLD 2019 focuses on eosinophil blood count and GesEPOC on a definition of asthma-COPD overlap. Future research should focus on the identification of individual therapeutic response. This knowledge will allow us to advance in precision medicine in COPD and provide the best therapeutic approach for the individual patient.
Conflicts of interestJLLC has received honoraria over the last three years for lecturing, scientific advice, participation in clinical studies or writing for publications for (alphabetical order): AstraZeneca, Boehringer Ingelheim, Chiesi, CSL Behring, Esteve, Ferrer, Gebro, GlaxoSmithKline, Grifols, Menarini, Novartis, Rovi and Teva. JJSC has received fees as speaker of AstraZeneca, Boehringer Ingelheim, Bial, Ferrer, Esteve, Menarini, Mundipharma, Novartis, Rovi and TEVA; Consulting fees for AirLiquide, AstraZeneca, Boehringer Ingelheim, Chiesi, Ferrer, GlaxoSmithKline, Esteve, Mundipharma, and Novartis and research grants from GlaxoSmithKline and Boehringer Ingelheim. MM has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, AstraZeneca, Menarini, Rovi, Bial, Zambon, CSL Behring, Grifols and Novartis, consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Bial, Gebro Pharma, CSL Behring, Laboratorios Esteve, Ferrer, Mereo Biopharma, Verona Pharma, TEVA, pH Pharma, Novartis and Grifols and research grants from GlaxoSmithKline and Grifols.