Metastatic cutaneous melanoma is a cancer that accounts for only 4% of all skin tumors, but one that confers high morbidity and mortality.1 It causes approximately 5% of secondary malignancies of the lung, yet only 2% of patients with thoracic metastases develop pleural effusions.2 This case is of interest given the unusual primary presentation and the fact that the histological diagnosis was based on a sample of pleural tissue.
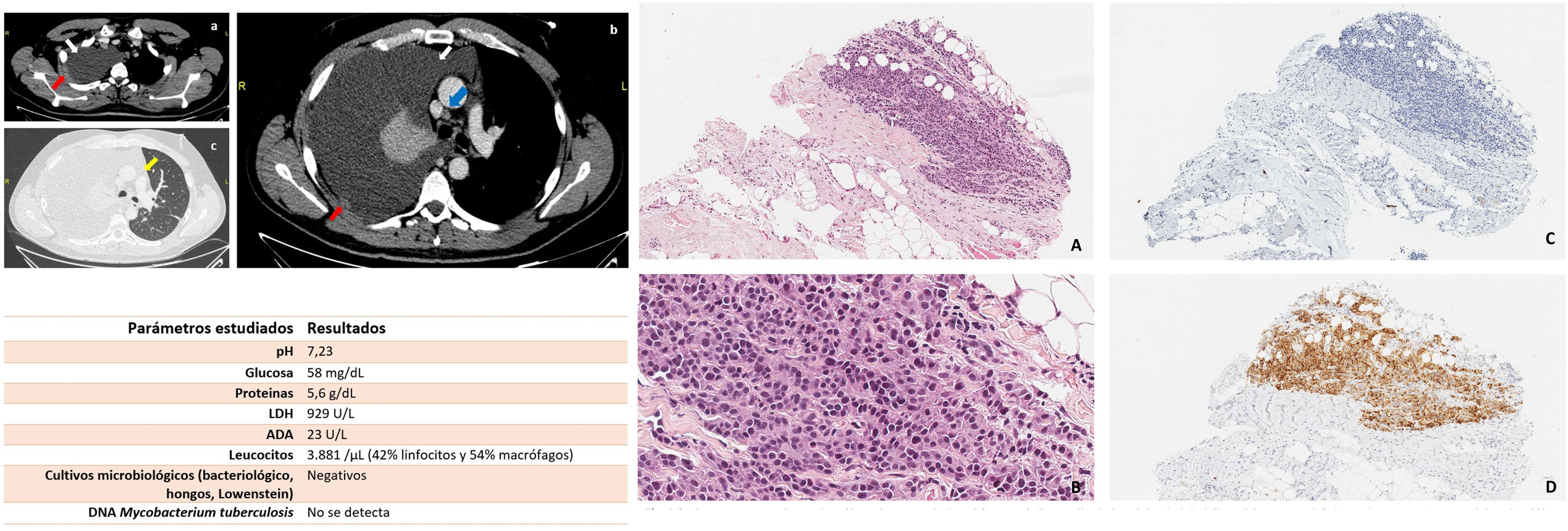
We report the case of a 32-year-old man with no drug allergies, no recurrent infections in childhood, and no significant medical or surgical history. He was admitted to the respiratory medicine department for large right pleural effusion associated with progressive dyspnea on exertion and pleuritic pain. Contrast-enhanced chest computed tomography (CT) revealed right tension hydrothorax with focal areas of nodular thickening in the parietal pleura (Fig. 1.1), lymphadenopathies in the right cardiophrenic angle, and right mammary, hilar, right paratracheal, infracarinal and bilateral prevascular chains. Abdominal and pelvic CT showed retrocaval, intercaval-aortic and preaortic lymphadenopathies and lytic lesions in L5 and S3. Brain magnetic resonance imaging was significant for multiple punctiform bone lesions in the frontal and parietal bones. HIV serologies and hepatotropic viral testing were negative.
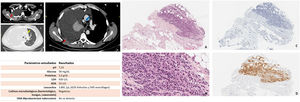
(1.1) Chest computed tomography images. (A and B) Mediastinal window. (C) Parenchymatous window. Massive right pleural effusion (white arrows) associated with focal pleural thickening (red arrows), mediastinal structures shifted to the left (yellow arrow), and right paratracheal mediastinal lymph nodes (blue arrow) are observed. (1.2) Results of the study of pleural fluid obtained by thoracentesis. (1.3) Panoramic image of pleural tumor infiltration (H–E 20×). (B) Detail of medium-sized plasmacytoid cell types and lateralized basophil nuclei, occasional nucleoles, and scant or moderate cytoplasms arranged in threads, strings or diffusely (H–E 40×). (C) Absence of epithelial marker expression (CK AE1-3 10×). (D) Intense diffuse cytoplasmic expression to melanocytic markers (HMB-45 10×).
Chest ultrasound showed a large collection of free anechoic pleural fluid in the right hemithorax. A total of 1200ml of hematic serous exudate-type fluid was extracted for diagnostic and therapeutic thoracentesis (Fig. 1.2). The study was completed with a percutaneous pleural biopsy (Fig. 1.3). The results were consistent with a melanocytic lesion, so the studies were extended to rule out a metastatic origin.
Dermatological assessment and ophthalmological studies were performed, ruling out lesions suggestive of melanoma. The genetic study did not show BRAF mutations that are typically related with more aggressive disease with a high metastatic potential.
He was referred to the medical oncology department where treatment with immunotherapy was initiated (4 cycles of nivolumab and ipilimumab). Despite treatment, the patient's general condition deteriorated. He was admitted twice for respiratory sepsis and died 3 months after diagnosis.
Metastatic melanoma is a rare entity1,2 in which a mucosal-visceral origin should be considered in the absence of identifiable lesions. Diagnosis is difficult, and the prognosis is usually worse than in cutaneous melanoma.4,5 It is known to be associated with lung metastases, but data on pleural metastases are scant.1,2 Pleural metastases alone are considered a poor prognostic factor with a 1-year survival of 33%.3 Early diagnosis may improve prognosis, although there is insufficient evidence to confirm this. In our patient, pleural biopsy – in the absence of other detectable locations – was essential for histological diagnosis and the initiation of oncological treatment.
In a patient with pleural melanoma, the primary site should be re-evaluated to differentiate between primary and secondary melanoma. This can be complicated in clinical practice, but the pleural location should be taken into consideration despite its rarity.