Fifty-five years ago, the Union for International Cancer Control (UICC) published a brochure with the classification of the anatomic extent of lung cancer based on three components: the primary tumour, the nodal spread and the distant metastases – the TNM classification. Pierre Denoix, a surgical oncologist form Paris, had developed this classification in a series of articles that he published between 1943 and 1952. The UICC adopted it in 1960 and the American Joint Committee on Cancer (AJCC), in 1977. Since then, both institutions have been in charge of promulgating and revising the classification.1 That first edition was followed by five more, the revisions of which were informed by the analyses of a North American database of over 5000 patients managed by Clifton F. Mountain. Useful as it was, that database originated from a single geographic area, and all therapeutic modalities other than surgery were underrepresented. These limitations prompted the International Association for the Study of Lung Cancer (IASLC) to create a large international database of patients with lung cancer from as many countries as possible and treated by all types of therapies with the objective to revise futures editions of the TNM classification of lung cancer. This mission was undertaken by the IASLC Staging and Prognostic Factors Committee (SPFC), created in 1998, under the leadership of its first Chair, Peter Goldstraw, a thoracic surgeon from London.2 To their credit, the Bronchogenic Carcinoma Cooperative Groups I, II and III, organized within the Thoracic Oncology Area of the Spanish Society of Pneumology and Thoracic Surgery (SEPAR, in its Spanish acronym), have contributed thousands of patients to the three phases of the IASLC Staging Project with high quality data, including molecular information for the present phase of the Project: 2993 patients for the 7th edition; 2362 for the 8th; and, up to 28th February 2021, 1226 for the 9th.
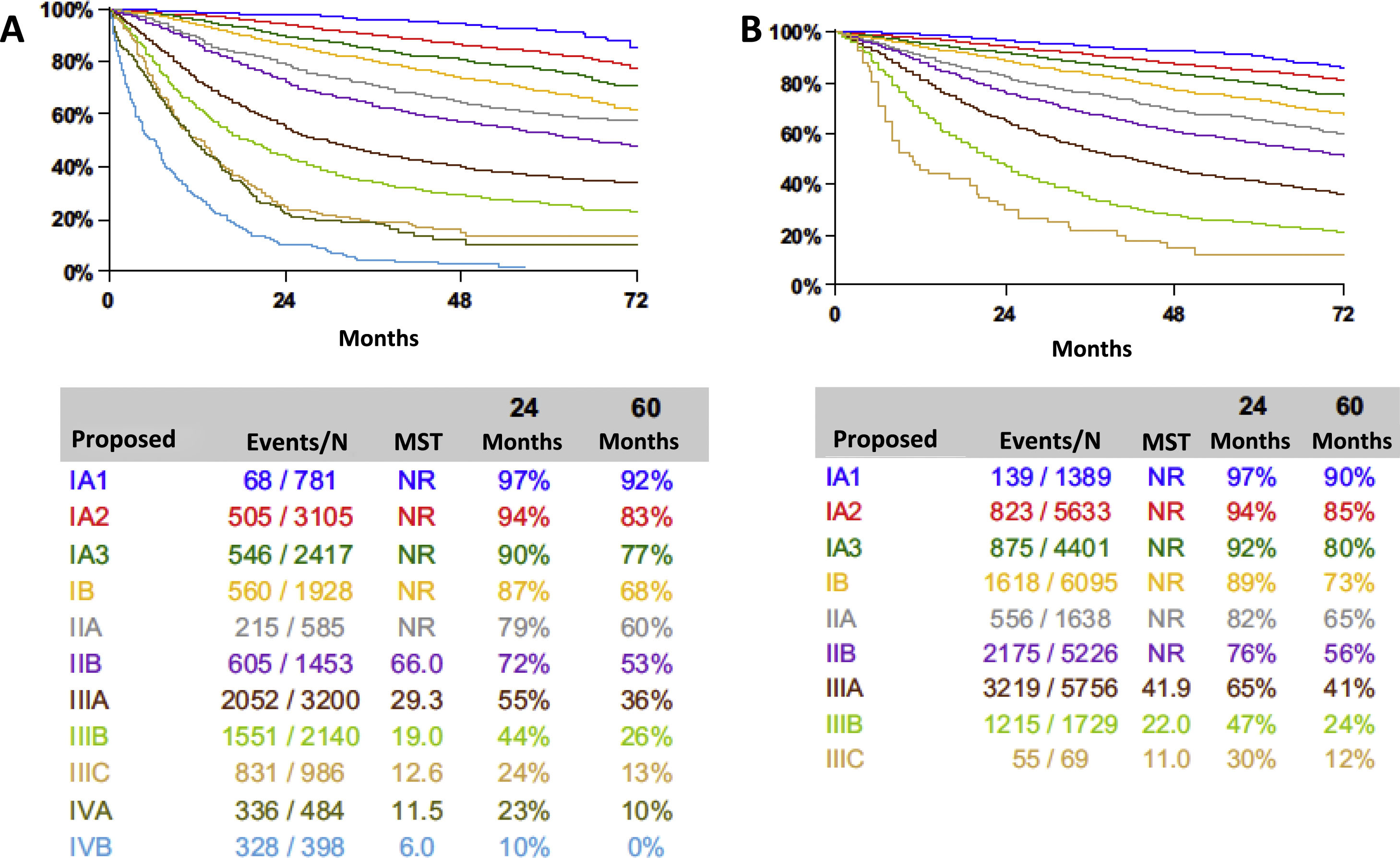
In the past two decades, the IASLC SPFC has collected two large databases of patients with lung cancer submitted from many countries and treated by different therapeutic modalities.3,4 Their analyses allowed the evidence-based recommendations for changes that were subsequently introduced in the 7th and the 8th editions of the TNM classification of lung cancer. In essence, the analyses showed the prognostic relevance of tumour size; allowed the reassignment of some descriptors to different T categories; highlighted the prognostic value of quantifying nodal disease, either by considering the number of involved nodal zones or of involved nodal stations; and showed the relevance of the number of metastatic sites and their anatomic location.5 However, these innovations hardly increased the power of prognostication of the TNM classification as measured by the R2 statistic. The R2 statistic for the clinical and pathologic stage groupings of the 7th edition was 67.5 and 45.7, respectively; and that for the clinical and pathologic stage groupings of the 8th edition, 68.3 and 46.9, respectively.6 Although some gain was achieved in the 8th edition, there is a big portion of prognosis that is not explained by the classification of anatomic extent of lung cancer (Fig. 1).
At the time of this writing, cases still are being submitted for the analyses that will lead to the 9th edition of the classification, due to be published in 2024. From the discussions in the various subcommittees of the IASLC SPFC, it is reasonable not to expect more T categories based on smaller tumour size fractions that would make the classification impractical. However, some type of quantification of nodal disease may be possible if there are enough data. The problem with the quantifications proposed in the previous two editions7,8 is that they derived from pathologic staging of tumours that had an adequate intraoperative nodal assessment. When quantification is analysed at clinical staging, it does not work, because the available tests to study nodal disease preoperatively are not so accurate as a properly performed systematic nodal dissection. One potential way to solve this problem would be to keep the present N categories for clinical staging and introduce some kind of quantification to define the pathologic N categories. Regarding the M component of the classification, it would be desirable to further study the impact of organ-specific metastases and to validate the definition of oligometastatic disease.9
What can we expect from the forthcoming 9th edition? In order to enhance the prognostic power of the TNM classification, the main innovation in the data being collected now is the inclusion of molecular information: genetic biomarkers, copy number alterations and protein alterations.10 All these will be combined with clinical, biological and anatomic factors to create prognostic groups that will increase our capacity to refine prognosis and, hopefully, to design a therapeutic plan in a more personalized way.11 The TNM classification is a strong prognosticator, but the present trend towards personalized diagnosis and treatment requires going beyond what the TNM classification can offer.
The identification of cancer cells or their circulating DNA and RNA by means of liquid biopsy is not included in the TNM classification, but reveals tumour extension into the circulatory system. Their presence after treatment completion is associated with a worse prognosis.12 In future editions of the TNM classification, the results of liquid biopsy may play a role in complementing it. In fact, it already has been suggested to add the letter B, for blood, to the TNM to describe the presence of cancer material in the blood.13 Other cancer cells that should be included in future editions of the classification are those spread through air spaces, that have been identified in all types of lung cancer and are associated with a worse prognosis.14,15
According to the rhythm in which new data are being submitted, there is every reason to think that the planned analyses will be possible and that, although no major changes can be expected in the pure anatomic classification, when combined with other prognostic factors, clinical management of patients with lung cancer will be much improved.