Large airway collapse (LAC) is mainly caused by tracheobronchomalacia (TBM) and excessive dynamic airway collapse (EDAC).1 It is defined by a decrease of at least 50% in the cross-sectional area of the tracheobronchial lumen.1,2 TBM is characterised by a weakness of the tracheobronchial cartilaginous wall, while EDAC is defined by the presence of excessive bulging of the posterior membrane into the airway lumen,3–5 without anomalies in the cartilaginous structures.3 LAC is an underdiagnosed condition, especially EDAC, with nonspecific symptoms that can mimic other respiratory diseases, such as asthma. Symptoms are shortness of breath, cough, stridor, respiratory infections and difficulty in expectorating.
Severe asthma accounts for 5% of patients with asthma.6 While TBM is a condition related with severe asthma, the association between asthma and EDAC has not been described.7,8 Our objective was to study the prevalence of LAC and EDAC in patients with severe asthma, and to analyse the associated factors.
This was an observational prospective multicentric study conducted in the Respiratory Departments of Hospital Sant Pau (Barcelona) and Hospital Germans Trias i Pujol (Badalona) between years 2017 and 2019. Twenty patients with severe asthma consecutively selected in Asthma Units, over 18 years old without exacerbations for at least the previous four weeks were included. Patients with other respiratory conditions, with mild-to-moderate asthma, previously diagnosed tracheal conditions or with previous tracheostomy were excluded.
Demographic, anthropometric and clinical data were collected. Clinical data included questionnaires about symptoms (Asthma Control Test) and quality of life (Sydney Asthma Quality of Life Questionnaire), asthma treatment, exacerbations during the previous year, pulmonary function and laboratory determinations. A flexible bronchoscopy (FBC) was performed under mild sedation, using a bronchoalveolar lavage (BAL) in medium lobe or lingula bronchi for microbiological cultures and cell count. Bronchoscopy images were videorecorded in order to calculate de degree of the collapse. LAC was diagnosed when a difference of at least 50% in the cross-sectional area of the lumen between inspiration and expiration at tidal volume was seen in one or more of the following locations: trachea, right main bronchus or left main bronchus. Study protocol was approved by Ethics Committee at our institution (PI-16-174), and all subjects gave their informed consent. Quantitative variables were described using median (interquartile range), and qualitative variables were described using absolute and relative frequencies. Comparisons were conducted using Mann–Whitney U in the case of quantitative variables, and Fisher's exact test for qualitative variables. Those variables with p<0.05 were included in a univariate analysis using logistic regression. Calculations were performed using SPSS v22 (Chicago, IL, USA).
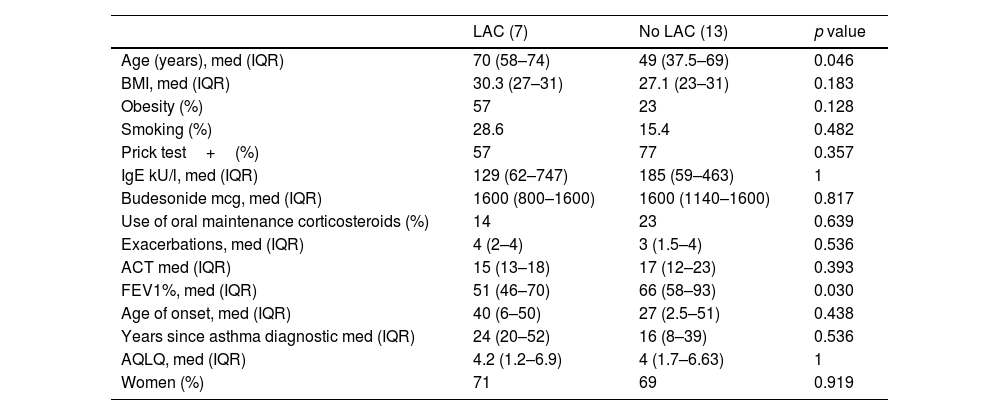
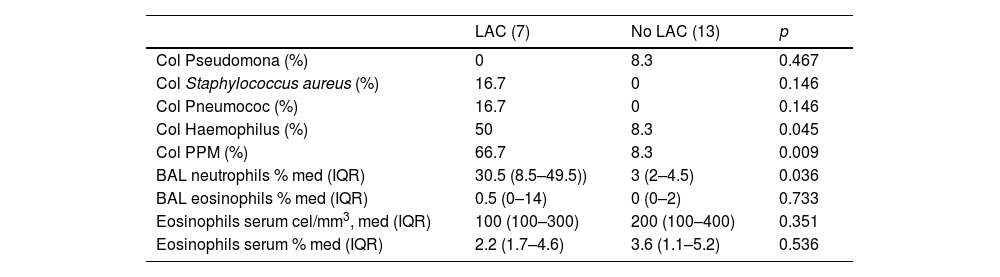
Seven of our 20 severe asthmatic patients (35%) had LAC, 6 of them (30%) EDAC and one patient TBM (5%). Main bronchi was the most frequent location (5/7). Patients with LAC were older (70 years vs 49 years, p=0.046) and had lower pulmonary function (FEV1%; 51 vs 66, p=0.03), with neutrophilia in bronchoalveolar fluid (30.5% vs 3%; p=0.03) and were more likely to be colonised by potentially pathogen microorganisms (PPM) (66.7% vs 8.3%, p=0.009) especially Haemophilus spp. (50% vs 8.3%, p=0.04). These patients had more symptoms and had a higher body mass index (BMI), without statistical significance. No differences in other variables were found (Tables 1 and 2). In the univariate logistic analysis, only colonisation with PPM was associated with an increased risk for LAC (OR 22 (1.54–314); p=0.023).
Demographic, Anthropometric and Clinical Characteristics.
| LAC (7) | No LAC (13) | p value | |
|---|---|---|---|
| Age (years), med (IQR) | 70 (58–74) | 49 (37.5–69) | 0.046 |
| BMI, med (IQR) | 30.3 (27–31) | 27.1 (23–31) | 0.183 |
| Obesity (%) | 57 | 23 | 0.128 |
| Smoking (%) | 28.6 | 15.4 | 0.482 |
| Prick test+(%) | 57 | 77 | 0.357 |
| IgE kU/l, med (IQR) | 129 (62–747) | 185 (59–463) | 1 |
| Budesonide mcg, med (IQR) | 1600 (800–1600) | 1600 (1140–1600) | 0.817 |
| Use of oral maintenance corticosteroids (%) | 14 | 23 | 0.639 |
| Exacerbations, med (IQR) | 4 (2–4) | 3 (1.5–4) | 0.536 |
| ACT med (IQR) | 15 (13–18) | 17 (12–23) | 0.393 |
| FEV1%, med (IQR) | 51 (46–70) | 66 (58–93) | 0.030 |
| Age of onset, med (IQR) | 40 (6–50) | 27 (2.5–51) | 0.438 |
| Years since asthma diagnostic med (IQR) | 24 (20–52) | 16 (8–39) | 0.536 |
| AQLQ, med (IQR) | 4.2 (1.2–6.9) | 4 (1.7–6.63) | 1 |
| Women (%) | 71 | 69 | 0.919 |
LAC: large airway collapse; BMI: body mass index; ACT: Asthma Control Test; AQLQ: Asthma Quality of Life Questionnaire; med: median; IQR: interquartile range.
Microbiological and Inflammatory Characteristics.
| LAC (7) | No LAC (13) | p | |
|---|---|---|---|
| Col Pseudomona (%) | 0 | 8.3 | 0.467 |
| Col Staphylococcus aureus (%) | 16.7 | 0 | 0.146 |
| Col Pneumococ (%) | 16.7 | 0 | 0.146 |
| Col Haemophilus (%) | 50 | 8.3 | 0.045 |
| Col PPM (%) | 66.7 | 8.3 | 0.009 |
| BAL neutrophils % med (IQR) | 30.5 (8.5–49.5)) | 3 (2–4.5) | 0.036 |
| BAL eosinophils % med (IQR) | 0.5 (0–14) | 0 (0–2) | 0.733 |
| Eosinophils serum cel/mm3, med (IQR) | 100 (100–300) | 200 (100–400) | 0.351 |
| Eosinophils serum % med (IQR) | 2.2 (1.7–4.6) | 3.6 (1.1–5.2) | 0.536 |
LAC: large airways collapse; med: median; IQR: interquartile range; PPM: potentially pathogenic microorganisms.
In our study, prevalence of LAC was 35% in patients with severe asthma, mainly EDAC. These patients were older, with worse pulmonary function, more respiratory symptoms, more neutrophil count in bronchoalveolar fluid, and with higher prevalence of colonisation by PPM.
Prevalence of LAC is lower than in the only previous study performed exclusively in asthmatic patients. Dal Negro and cols evaluated 202 asthmatic patients and found an increase in the prevalence related to asthma severity. Prevalence of EDAC and TBM measured endoscopically was 69.2% and 18.4%, respectively.9 This difference in prevalence between the two studies could be related to the differences in gender, mean pulmonary function differences (68.5% females and FEV1% 48.5 in Dal Negro’ study) and the smaller sample size of our study. Recently, Cosío et al., in 100 uncontrolled severe asthmatics found lower prevalence of LAC than ours; 3%.10 However, the studies are not comparable as the objectives were clearly different. Other studies focusing on COPD patients have described prevalence of LAC between 9 and 70%.1 The use of different techniques to define LAC (FBC, CT scan or magnetic resonance) could be the reason of such variability, as well as the difference in the degree of collapse used to define LAC, or the respiratory manoeuvre required to the patient. We believe that the FBC is the best method to evaluate LAC in severe asthma, since it adds information on the characteristics of the airway, cellularity, inflammation or microbiological data.
Our patients with LAC had a higher BMI. The increase on collapsibility in obese patients could be explained by increased intrathoracic pressure that facilitates collapse of the airways at the end of expiration, especially when airway walls are damaged.11 Boiselle et al. described that COPD patients with BMI over 35 had a mean tracheal collapse of 69%, while in those with BMI below 35 mean tracheal collapse was only 5%.12
In our study patients with LAC had poorer pulmonary function, which is not in line with previous studies in patients with COPD,13–15 where no relation was found between LAC and FEV1.15 On the other hand, Fischer et al.,16 described that FEV1 was 18% lower in children with cystic fibrosis when TBM was present. In the same study, the authors found an increase in the prevalence of infection by Pseudomonas aeruginosa in patients with TBM.16 In our study we observed an increase in the colonisation by PPM, especially Haemophilus influenza; to our knowledge is the first paper that it has been described in patients with LAC and severe asthma.
The damage to the bronchial wall associated with LAC in asthmatic patients remains unclear but could be explained by different mechanisms, such as asthmatics are more prone to infections,17 their high levels of elastase derived from neutrophilic inflammation,18 the use of high dose of inhaled corticosteroids19 or the high intrathoracic pressure due to chronic cough.15,20 On the other hand, an increase in PPM colonisation and infection16 could be the result of the impaired drainage of mucus due to bronchial collapse. Our data showing bacterial colonisation and neutrophilia in these patients would support part of mechanisms discussed.
The article suffers from some limitations such as the sample size or not collecting follow-up data, which we believe are counteracted by the originality of the findings.
LAC, especially EDAC, is highly prevalent in patients with severe asthma, and it is associated with increased symptoms, worse pulmonary function and a higher risk of colonisation by PPM. Thus, it is a comorbidity which should be considered in patients with poor clinical control. Increased colonisation by PPM has not been previously described in patients with LAC and asthma. Whether this colonisation is a risk factor for LAC, or its consequence, is still to be elucidated. Studies with larger samples and a follow-up must be carried out.
FundingFunded by a grant from Fundació Catalana de Pneumologia (FUCAP), 2018.
Conflict of InterestsThe authors state that they have no conflict of interests.