Introduction
Schistosomiasis produced by Schistosoma mansoni is an endemic disease in the Arabian Peninsula, Africa, northern South America, and the Caribbean. In some countries, like Brazil, it constitutes a public health problem affecting thousands of people. In Venezuela the estimated prevalence, based on an examination of feces in endemic zones, is 1.39%. However, more sensitive diagnostic methods show a greater prevalence (enzyme-linked immunosorbent assay [ELISA] on soluble egg antigen [SEA], 20.6%).
There have been reports in the last several years of the presence of S mansoni eggs in the lungs of patients with chronic visceral disease.1 These eggs act as emboli obstructing the pulmonary arterioles but generally not affecting capillaries. Autopsy studies have indicated that the principal changes caused by S mansoni eggs are the appearance of fibrin deposits and marked hyperplasia of endothelial cells in the small arteries and arterioles. The formation of complex fibrin thrombi and revascularization, followed by congestion and the focal dilation of blood vessels with plexiform lesions, have also been described.1 The inflammatory process heals by fibrosis, which causes narrowing, thickening, and occlusion of the pulmonary arterioles.
Although treatment with praziquantel can effectively eradicate infection by S mansoni with minimal toxicity, certain functional abnormalities will probably persist in view of the fibrotic changes that may leave residual lesions in the small pulmonary vessels with minimal functional alterations.2
Physiological measurements during exercise are considered a useful diagnostic tool in evaluating patients with chronic obstructive pulmonary disease.3-9 Abnormal values are generally associated with alterations related to low cardiac output and gas exchange anomalies. The progressive cycle ergometer test (PCET) can contribute useful information for evaluating patients with such problems.3-9 It has also been suggested that this test offers greater sensitivity than others in measuring a series of parameters in patients with minimal functional abnormalities that cannot be identified with functional tests carried out at rest.
The literature to date does not supply adequate information on cardiopulmonary impairment and pulmonary vascular occlusive disease in schistosomiasis.1,2,10-14 For this reason our study was designed to evaluate cardiopulmonary response to exercise in patients successfully treated with praziquantel for chronic schistosomiasis with no clinical evidence of cardiopulmonary impairment and to determine the existence of functional abnormalities (oxygen consumption [VO2], oxygen transport, or gas exchange) compatible with the obstruction of the pulmonary vascular bed.
Patients and Method
Patient Selection
We studied 9 patients with a confirmed diagnosis of chronic schistosomiasis from the service specializing in schistosomiasis at the institute of tropical medicine at the Universidad Central de Venezuela. Four of the patients had signs of hepatosplenic involvement, 1 with signs of portal hypertension. The rest were asymptomatic and had received medical treatment 20 years earlier. None of the patients had clinical signs of cardiopulmonary abnormalities and all had given written consent to participate in the study. We also studied 10 healthy subjects from the same age group.
After epidemiological diagnosis of contact with waters potentially infested with cercariae of the parasite, patients who had tested serologically positive for S mansoni infection underwent clinical, parasitological, and immunological evaluation. The parasitological tests were based on evidence of the presence of parasite eggs in the patients' feces. Our populations had low parasite loads, which accounted for the fact that the number of eggs per gram of feces was low, making diagnosis by the Kato-Katz or any other concentration method difficult. For this reason we also did 2 immunological assays: ELISA-SEA and circumoval precipitin tests. The latter is a highly sensitive and specific indicator of parasite activity, which diminishes or may even become negative after medical treatment. Patients with S mansoni eggs in their stool or with a positive circumoval precipitin test (>10%) were included as cases of schistosomiasis.
All patients diagnosed with schistosomiasis with associated cardiopathology (ischemic, hypertensive, myocardiopathic, valvular, etc), pulmonary disease with involvement of the pleura (chronic obstructive pulmonary disease, asthma, bronchiectasis, cancer, tuberculosis, interstitial disease, etc), a history of chest surgery, neuromuscular or osteoarticular disease, obesity, or any other medical problem that could interfere with the stress test were excluded from the protocol.
Lung Function Tests
Simple spirometry was performed with a MedGraphics CardiO2 System spirometer (MedGraphics CardiO System, St. Paul, MN, USA). Forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and the FEV1/FVC ratio were calculated following the recommendations of the American Thoracic Society.15 The results were expressed as absolute values and percentage of predicted values.16 Carbon monoxide diffusing capacity was measured with the standard technique. Maximal voluntary ventilation was obtained directly using the 12-second maneuver and was used in the stress test as the maximal ventilatory capacity.
Cardiopulmonary Stress Test
The PCET was administered with an ergometric cycle (MedGraphics CardiO2 System, St. Paul, MN, USA) using the standard ramp protocol. The protocol begins with 2 minutes of rest, followed by a 3-minute period of pedaling with no resistance, and finally with a progressive increase in the work load (ramp) at a rate of 25 W/min.3-6 Patients and control subjects were encouraged to continue pedaling until symptoms limited their ability to exercise. The end of the test was determined by the appearance of symptoms, by the fact that submaximal heart rate (HR) had been reached, by a respiratory quotient more than 1.15, or by the presence of electrocardiographic abnormalities. HR and heart rhythm were monitored with a 12-lead electrocardiograph. Blood pressure was measured noninvasively with a pressure cuff connected to a mercury sphygmomanometer.
Minute ventilation (VE) and its components were measured using a pneumotachograph (MedGraphics CardiO2 System, St. Paul, MN, USA). Oxygen concentration was analyzed with a zirconium dioxide cell oxygen analyzer and expired carbon dioxide with an infrared ray carbon dioxide analyzer (MedGraphics CardiO2 System, St. Paul, MN, USA). The measurements and flow signals were electronically integrated by a computerized system that averaged VE, tidal volume, respiratory frequency, VO2, carbon dioxide production, and respiratory quotient every 30 seconds.
HR was used to evaluate HR reserve and oxygen transport indices (oxygen pulse and ΔHR/ΔVO2). Predicted maximal oxygen consumption (VO2max) was calculated in accordance with the recommendations of Jones and Campbell.17 The anaerobic threshold was determined noninvasively using the modified V-slope method.18 Maximum predicted HR was calculated with the following formula: maximum HR=220-age. Arterial oxygen saturation was monitored noninvasively with a fiber optic digital transducer (Ohmeda Biox 3740 pulse oximeter, Miami, FL, USA). Blood samples to measure arterial gases were taken at rest and immediately after exercise in order to evaluate pulmonary gas exchange and the dead space (VD) to tidal volume (VT) ratio.
Intensity of exercise-induced dyspnea, evaluated at rest and at the end of exercise, was quantified using the Borg scale.19
Echocardiographic and Doppler Evaluation
An ultrasound machine with transducer (Hewlett Packard, model 5500 or 1800) was used, which allowed us to obtain and record echo-Doppler, M-mode, and 2-dimensional (2D) echocardiographic signals. The study was carried out on all patients diagnosed with schistosomiasis to rule out the existence of other cardiovascular diseases that could cause alterations in functional tests.
Analysis of the M-mode and 2D signals included the following parameters: diastolic diameters of both ventricles, right ventricular wall, interventricular septum and posterior left ventricular wall, area of the left and right atria, and tricuspid annular plane systolic excursion to calculate right ventricle ejection fraction. Aortic root and pulmonary artery diameters were also measured
The analysis of the velocity signals obtained by continuous Doppler allowed us to estimate systolic blood pressure, which was calculated by measuring the maximal tricuspid regurgitation flow velocity (TRVmax) and applying the following formula:
(TRVmaxx4)+right atrial pressure
Right atrial pressure was estimated from the 2D image of the degree of inferior vena cava inspiratory collapse (>50%= 5 cm H2O; <50%=10 cm H2O; 0%=5 cm H2O). Diastolic pulmonary artery pressure was obtained from the recording of the pulmonary valve regurgitation gradient at the end of diastole with continuous Doppler using the following formula:
(end-diastolic velocityx4)+right atrial pressure
Systolic pulse and Doppler velocity curve analyses included the following variables: right ventricular ejection time, from the onset of right ventricular ejection until right ventricular ejection was zero; isovolumetric contraction period or right ventricular pre-ejection period, from the onset of the QRS complex of the electrocardiogram until the onset of right ventricular ejection; pulmonary acceleration time, from the onset of pulmonary ejection until peak flow velocity was reached. Mean pulmonary pressure was also calculated using the following formula:
79-(0.45xright ventricular acceleration time)
Statistical Analysis
The results of lung function tests, PCET, and echocardiography were expressed as means (SD). To compare the results of anthropometric data, lung function tests, and PCET between patients and control subjects the Student t test for independent samples was used. A level of statistical significance with a probability value less than .05 (P<.05) was accepted. The results are presented in the tables and figure.
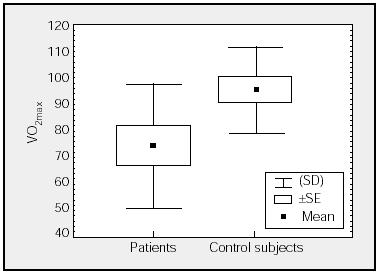
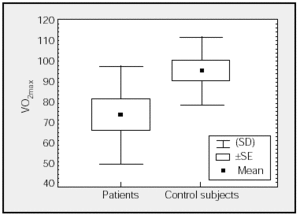
Figure. Comparison of maximal oxygen consumption (VO2max) (percentages) of patients and control subjects.
Results
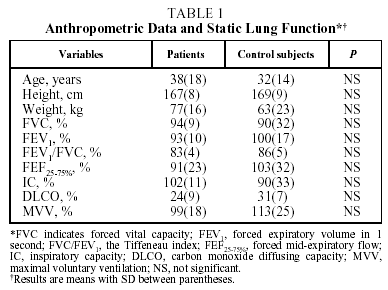
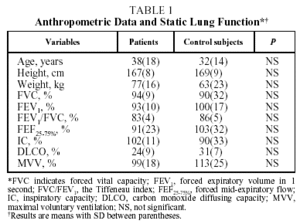
Mean anthropometric data and static lung function findings for patients and control subjects are detailed in Table 1. The mean ages of patients (38[18] years) and control subjects (32[14] years) were similar. As can be observed in Table 1, lung function, including carbon monoxide diffusing capacity, was similar in both groups, indicating that patients with schistosomiasis had normal resting lung function.
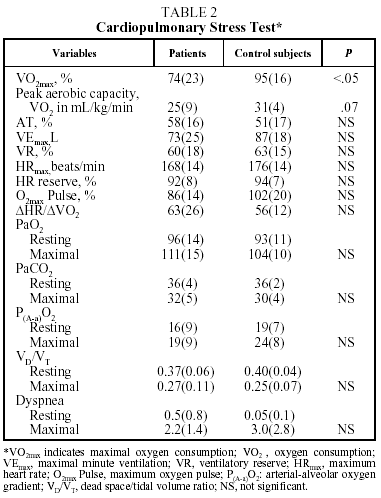
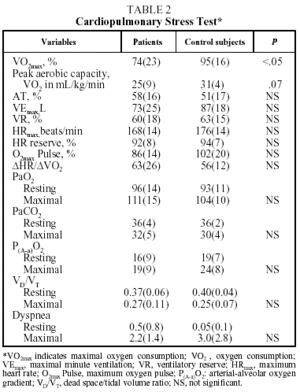
Table 2 shows mean values for the PCET. This table and the figure show that patients had a slightly lower exercise capacity (VO2max:74%[23%]) than did control group subjects (VO2max:95%[16%]). The stratification of the degree of functional impairment on the basis of peak aerobic capacity (VO2 in mL//kg/min), at 25[9] mL/kg/min, showed that patients had a minimal decrease in exercise tolerance (functional class A: VO2/kg≥20 mL/kg/min).20,21
Exercise intolerance in patients was related to signs of cardiovascular limitations or physical detraining, and was characterized by a decrease in the recruitment of HR reserve (92%[8]%) for the amount of effort exerted (VO2max:74%[23]%).3-6 It was also observed that the anaerobic threshold (58%[16]%) and oxygen transport indices (maximum oxygen pulse and ΔHR/ΔVO2) of the patients were similar to those of the control subjects. Nor were differences observed between patients and control subjects in ventilatory parameters (maximal minute ventilation [VEmax], VEmax/minimum voluntary ventilation), gas exchange indexes (PaO2, PaCO2, arterial-alveolar oxygen gradient, VD/VT), or shortness of breath during exercise.
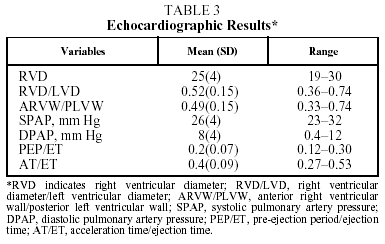
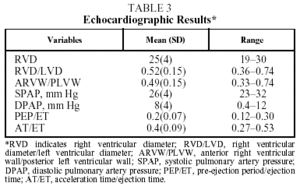
The echocardiographic results in Table 3 show no alterations that would indicate the presence of heart disease associated with pulmonary artery hypertension.
Discussion
Ours is the first controlled study to compare changes in exercise tolerance and cardiopulmonary response to exercise between patients with no evidence of cardiopulmonary impairment treated with praziquantel for chronic schistosomiasis and healthy individuals of similar age. The most important findings were a) resting lung function including carbon monoxide diffusing capacity of patients with chronic schistosomiasis was normal; b) evidence of minimal cardiovascular limitation and normal respiratory response indicated that patients had only a slight decrease in exercise capacity, corresponding to functional class A; and c) no echocardiographic abnormalities that might indicate the presence of pulmonary hypertension or pulmonary vascular occlusive disease were observed.
There has been little information published on lung function and pulmonary complications in patients with chronic schistosomiasis with or without cor pulmonale.1,2,10-14
Some studies show histological evidence of fibrin deposits and marked hyperplasia of endothelial cells in the small pulmonary arteries and arterioles with complex fibrin thrombus formation and revascularization in patients with schistosomiasis and cor pulmonale.1 However, no study has been carried out that would determine histological alterations in the pulmonary vascular bed of patients with schistosomiasis but without cor pulmonale or in those who have received successful medical treatment. As this inflammatory process heals by fibrosis, it is thought that these changes are irreversible with treatment and that residual fibrotic lesions capable of affecting pulmonary vascular function may remain.
Frayser and Alonso10 observed that maximal voluntary ventilation and FEV1 decreased slightly with no significant desaturation in stress tests compared to resting values in 14 chronic schistosomiasis patients in an uncontrolled study. However, the increase in carbon monoxide diffusing capacity after exercise of the patients was significantly lower than values used for reference (8.8 mL in the patients vs the reference of 16.4 mL).10 It is important to point out that such a difference is open to question, as the characteristics of the patients compared were quite dissimilar.
Lemle et al11 evaluated resting lung function in 5 patients with chronic schistosomiasis and cardiopulmonary complications. The results showed spirometric values within the normal range. The only abnormality reported was the presence of hypoxia due to an increase in right-to-left shunt in 2 patients with cyanosis. Unfortunately no echocardiographic studies were done to rule out the possibility of other associated diseases.
Until the present study, exercise tolerance and cardiopulmonary response to effort in patients with chronic schistosomiasis with no evidence of cardiopulmonary impairment had not been studied by direct measurement of VO2 and blood gas analysis. Our study represents the first such attempt and the results indicate that these patients have resting lung function similar to those of the control group. These findings differ from the assertion of Frayser and Alonso10 that maximal voluntary ventilation and FEV1 decrease. It is probable that the differences can be explained by methodological flaws in the data described by Frayser and Alonso,10 as they performed no measurements of the altered parameters in control subjects for comparison with patients' values.
The other findings of the present study were the changes observed in the PCET. The results indicated that patients with chronic schistosomiasis had a slight decrease in exercise tolerance compared to the control subjects. However, the patients as a group were functional class A, with an average VO2/kg/min of over 20 mL/kg/min (minimal cardiopulmonary limitation). This exercise intolerance is related to diminished HR reserve for the amount of effort exerted, indicative of cardiovascular limitation or physical detraining. It is impossible, however, to distinguish between these two possibilities with this test.3-6 The rest of the cardiovascular, oxygen transport, and respiratory parameters were similar to those of the control group. The presence in these patients of an anaerobic threshold, oxygen transport parameters (ΔHR/ΔVO2 and oxygen pulse), and gas exchange indexes (VD/VT, PaO2, arterial-alveolar oxygen gradient) similar to those of the control group makes the existence of pulmonary vascular occlusive disease in patients previously treated with praziquantel for chronic schistosomiasis unlikely.
It is difficult to compare our results with those of previous studies,10,11 as the methods of the studies and the clinical characteristics of the patients evaluated were very different. The present study included patients with no evidence of cardiopulmonary impairment treated with praziquantel, while the others evaluated either patients in advanced stages of the disease who did show evidence of cardiopulmonary impairment11 or who were untreated.10
In conclusion, the results of the present study indicate that patients treated with praziquantel for chronic schistosomiasis with no signs of cardiopulmonary impairment have normal resting lung function. However, exercise tolerance is slightly decreased, which is probably related to physical detraining. The data during effort show normal oxygen transport and gas exchange, which would make the existence of any type of pulmonary vascular disease in these patients unlikely. Abnormalities may appear in later stages of the disease or in patients who do not receive early successful medical treatment.
Acknowledgments
This study was carried out with the support of the National Council for Scientific and Technological Research (Consejo Nacional de Investigaciones Científicas y Tecnológicas), project S1-97001105.
Correspondence: Dra. M. Montes de Oca de Loyo.
CS 5150. P.O. Box 025323.
Miami, FL, 33102-5323. USA.
E-mail: jgloyo@telcel.net.ve
Manuscript received August 27, 2002.
Accepted for publication April 29, 2003.