To analyze the results obtained in a lung cancer (LC) screening program since its inception five years ago regarding correct referrals, diagnostic and therapeutic delay times and days of hospitalization. To compare the diagnostic–therapeutic delays and hospital stays with those obtained in patients evaluated with the standard system.
Patients and methodsIncluded for study were all those patients evaluated in our lung cancer screening program (LCSP) in the last five years. For the cases with LC, we recorded the dates the patients were referred to a specialist, the first consultation, diagnostic tests, stage, start of treatment, and days of hospitalization. We compared these same data with LC patients who did not partake in the LCSP and were diagnosed between October 2008 and October 2010.
ResultsWe evaluated 179 patients remitted to the LCSP, which represented 26.7% of the consultations; 166 (92.7%) of the referrals were correct, out of which 44.5% were LC. In 75.6% of these, the entire study was completed in the outpatient setting, and more than 85% of the cases met the current recommendations related with diagnostic–therapeutic delays. When these results were compared with the non-LCSP group (n=151), differences were found in the data for hospitalizations: there was a lower percentage of hospitalizations (P<.0001) and shorter hospital stays (P<.0001) in the LCSP group. There were no differences between the two groups for diagnostic or therapeutic delays.
ConclusionIn our setting, LC screening programs allow for cancer studies to be carried out in the outpatient consultations in a large percentage of cases, and within the time periods recommended by current guidelines. In spite of this fact, we have detected that these programs are underused.
Analizar los resultados conseguidos desde su creación hace 5 años en una consulta de diagnóstico rápido de cáncer de pulmón (CDR-CP) relacionados con el buen uso de la derivación, tiempos de demora diagnóstica y terapéutica, y días de estancia hospitalaria. Comparar las demoras diagnóstico-terapéuticas y estancias hospitalarias con las obtenidas en los pacientes evaluados mediante la sistemática habitual (NCDR-CP).
Pacientes y métodoSe ha incluido a todos los pacientes valorados en nuestra CDR-CP en los últimos 5 años. En los CP se han registrado las fechas de derivación al médico especialista, primera consulta, realización de pruebas diagnósticas, estadificación, inicio del tratamiento y días de hospitalización. Se han comparado estos mismos datos con los pacientes NCDR-CP diagnosticados en el periodo de octubre 2008 a octubre de 2010.
ResultadosSe evaluaron 179 pacientes remitidos a CDR-CP que representan el 26,7% de las consultas ofertadas, siendo 166 (92,7%) las remisiones correctas, de las que el 44,5% correspondieron a un CP; en el 75,6% de ellos se realizó todo el estudio de forma ambulatoria y más del 85% de los casos cumplían con las recomendaciones existentes relacionadas con las demoras diagnóstico-terapéuticas. Al comparar estos datos con el grupo NCDR-CP (n=151), se encontraron diferencias relacionadas con los datos de hospitalización: menor porcentaje de ingresados (p<0,0001) y menos días de estancia (p<0,0001) en el grupo CDR-CP. No existieron diferencias entre ambos grupos en las demoras diagnósticas y terapéuticas.
ConclusiónEn nuestro medio la consulta de diagnóstico rápido de cáncer de pulmón permite realizar, en un gran porcentaje de casos, todos los estudios de forma ambulatoria y en plazos de tiempo acordes con las recomendaciones existentes. Pese a ello, hemos detectado una acusada infrautilización de las mismas.
In 2007, 16000 men and 2500 women in our country died from LC. In the United States, it is already the tumor with the highest mortality in women and, according to data from the Spanish National Statistics Institute (Instituto Nacional de Estadística), it ranks third in Spain, although the rate of deaths grows 6% annually. The severity of the disease is seen in the disheartening 5-year total survival rate, which hardly reaches 15%.1–3
The complexity of LC patient management has increased in recent years as more diagnostic and therapeutic options become available. In order to provide proper health care, there is a necessity for an optimal coordination among several medical specialties. The traditional strategy of referring patients with suspicion of LC to the emergency department or sequentially to the consultations of multiple specialists usually results in health care that is often slow and poorly coordinated while distorting the social and family lives of patients and generating higher care costs.4,5
In 1998, the British Thoracic Society (BTS) published recommendations about the specific maximum time intervals for the diagnosis and treatment of patients with LC.6 In 2000, the UK National Health Service Cancer Plan established the objectives for providing accelerated care to patients with any type of cancer.7 That same year, the RAND Corporation published quality indicators centered on the time transpired from the first anomalous radiograph until the diagnosis, and from the diagnosis until treatment.8 In 2003, the American College of Chest Physicians communicated the recommendations for the practical organization of LC management in the United States.9 Recently, in our country, the Catalonian Health Services promoted the establishment of programs for fast diagnosis of lung, breast and colorectal cancer. The objective of these ambitious initiatives is that the time transpired from the first specialized consultation of a patient suspected of having cancer until their treatment is no more than 30 days in most cases.10
In recent years, some pulmonology departments have introduced in their health-care services, as an alternative to hospitalization, LCSPs in order to make access to the specialized consultations easier, reduce the diagnostic time and initiate treatment as soon as possible.10–14 Although almost all the published reports agree by pointing out that delayed diagnostic and therapeutic times do not influence the overall survival in LC,15,16 there is no doubt that quicker diagnosis and treatment avoid anxiety in both the patients and their family members that delays can cause, which has been shown in several international and national studies.17–21
The objective of the present study is to analyze the results from our rapid-access LCSP since its inception (data related with the adequate use of the consultation, diagnostic and therapeutic delay times and hospital stays) and to compare them with those obtained from a two-year period of LC management that followed the traditional system.
Patients and MethodsWe have carried out a descriptive observational study of all the patients that had been evaluated in our LCSP from January 2006 to October 2010. The patients had been referred to our unit by their primary care (PC) physicians, emergency services and other specialized care units other than pulmonology. All the departments’ staff who had the possibility of referring patients had been informed of the existence of the LCSP and of its protocol, defining suspicion of LC as the only reason necessary for referral (suspicious radiological study, hemoptysis with or without pathological radiology, pleural effusion or pneumonia with torpid evolution in a patient at risk).
Our department, with a reference population of somewhat more than 200000 people in the region of Pamplona and northern Navarra, started up the rapid-access LCSP at the end of 2005. Three pulmonologists are involved in the unit, each with a time period dedicated to LCSP patients within their weekly schedules. If there are no appointment requests 48h before the allotted LCSP time period, the specialists’ schedules are then completed with ordinary pulmonology consultation requests. With the aim of being able to reduce the diagnostic–therapeutic delay times and to shorten the length of hospitalization as much as possible, workflow protocols were agreed upon with all the departments involved, including the nuclear medicine service at a private health-care center where the positron emission tomographies (PET/CT) are done.
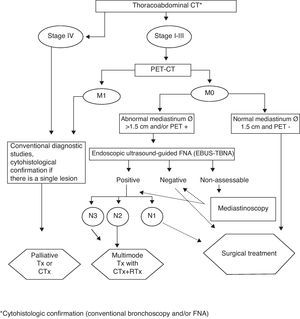
At the first LCSP visit, after anamnesis and simple chest radiography assessment, standard diagnostic tests are ordered and these patients are given priority. If LC is confirmed, the diagnostic–therapeutic algorithms of our LC are followed, in accordance with SEPAR (guidelines)22; Fig. 1 shows the flowchart used for non-small-cell LC. Up until October 2008, when in our department we began using real-time endobronchial ultrasound-guided transbronchial fine-needle aspiration (EBUS-TBNA), in all cases where mediastinal lymph node staging was considered necessary, we performed transbronchial fine-needle aspiration (FNA) without ultrasound guidance or, if necessary mediastinoscopy.
We designed a database compiling all the sociodemographic characteristics of the patients, departments from which they were referred, reason for the consultation, appropriateness of the consultation and final diagnosis. In the cases of LC, we recorded histologic type and clinical stage (TNMc) as well as delay times before LCSP, diagnostic testing and indicated treatment; in patients who were hospitalized, the number of hospitalization days was also recorded.
In the period from October 2008 to October 2010, we were able to obtain the same variables from all the LC cases diagnosed in our department by using the usual access system (non-LCSP), meaning without rapid-access LCSP (derived from ordinary pulmonology consultations, patients hospitalized due to emergencies, or patients treated in other departments that indicated their internal transfer to pulmonology due to a suspicion for LC) and these were compared with those obtained from the LCSP. In order to confirm the validity of this approach, in the LCSP group we verified the absence of statistically significant differences before and after October 2008, both in the diagnostic tests ordered (except EBUS-TBNA) as well as in the diagnostic–therapeutic delay times and hospitalization data.
The histologic classification was done according to the recommendations of the World Health Organization (WHO).23 For the tumor extension study (stage), as the cases had been diagnosed before the seventh classification by Mountain, we followed the sixth.24
For the statistical analysis, the G-Stat 2.0 statistical package was used. The degree of adjustment to normal distribution of the sample was determined with the Kolmogorov–Smirnov test. The quantitative data are expressed as means and standard deviation or as means and interquartile range, according to whether the distribution is normal or not. For the comparative analysis, the Mann–Whitney test was used in the quantitative variable and the χ2 was used to compare percentages. A P value=.05 was considered the limit of significance.
ResultsThe number of LCSP appointments available in the period analyzed was 670; 179 (26.7%) were used, and 166 of which (92.7%) were considered correct derivations. Table 1 shows the final diagnoses of the correctly referred patients, 44.5% of whom resulted with confirmation of LC. The origin of the patients was PC in 67.5% of the cases, specialized care in 31.2% and the emergency department in 1.3%.
Final Diagnoses in All the Cases Correctly Referred to the LCSP.
| Bronchopulmonary cancer | 74 (44.5) |
| Pneumonia with torpid evolution | 42 (25.3) |
| Benign lesions (hamartomas/granulomas) | 33 (19.8) |
| Pulmonary metastases | 13 (7.8) |
| Non-neoplastic hemoptysis | 2 (1.2) |
| Pleural effusion | 2 (1.2) |
| Total | 166 (100) |
From October 2008 to October 2010, 151 cases of LC were diagnosed in our department using the standard non-LCSP system; 32.4% were referred by PC, 38.5% by specialized care, and 29.1% by the emergency department. In this time interval, 30 LC were diagnosed by the LCSP system.
Within the LCSP group, we compared the LC evaluated during the first 3 years with those from the last 2, and we verified a lack of statistically significant differences in the diagnostic tests ordered (except EBUS-TBNA), the diagnostic–therapeutic delay and in the data referring to the hospitalization. Table 2 shows the comparison of the diagnostic–therapeutic delays in the LCSP group between the two time periods.
Comparison of the Diagnostic–therapeutic Delays in the LCSP Group During the First 3 Years and the Last 2 Years After the Incorporation of EBUS-TBNA.
| First 3 Years (n=44) | Last 2 Years (n=30) | P | |
| Interval in days between the 1st evaluation and staging: mean (interquartile range) | 13.5 (7–22) | 13.5 (6–27) | .90 |
| Interval in days between the first evaluation and the start of treatment: mean (interquartile range) | 28 (21–40) | 29 (20–39) | .75 |
| Interval in days between the referral date and staging: mean (interquartile range) | 21 (14–29) | 19.5 (12–30) | .68 |
| Interval in days between the staging date of the tumor and the start of treatment: mean (interquartile range) | 14 (8–22) | 12 (9–16) | .40 |
LCSP: patients evaluated in the rapid-access lung cancer screening program; EBUS-TBNA: real-time endoscopic ultrasound-guided transbronchial fine-needle aspiration.
Table 3 shows the sociodemographic data, diagnostic tests performed and the distribution by stages and histologic type of the cancers diagnosed in the LCSP and the non-LCSP. There is an observed absence of significant differences between both groups and a high percentage of LC diagnosed in advanced stage (78.3% LCSP and 71.5% non-LCSP).
Comparison of the Sociodemographic Data, Diagnostic Tests Done, Distribution by Stages and Histologic Type, Between LCSP and Non-LCSP.
| LCSP (n=74) | Non-LCSP (n=151) | P | |
| Sociodemographic data | |||
| Age: mean (SD) | 63.9 (11.1) | 64.2 (11.2) | .86 |
| Sex: % males | 81.0 | 77.5 | .53 |
| Diagnostic tests | n, % | n, % | |
| Work-up | 74 (100) | 151 (100) | |
| Respiratory function tests | 74 (100) | 151 (100) | |
| Simple chest radiography | 74 (100) | 151 (100) | |
| Chest computed tomography | 74 (100) | 150 (99.3) | .48 |
| Bronchoscopy | 54 (72.9) | 104 (68.8) | .52 |
| Transthoracic aspiration | 22 (29.7) | 41 (27.1) | .68 |
| PET | 33 (44.5) | 79 (52.3) | .27 |
| EBUS-TBNA | 9 (12.1) | 19 (12.5) | .92 |
| Distribution by stages | n, % | n, % | .58 |
| IA | 4 (5.4) | 13 (8.6) | |
| IB | 8 (10.8) | 11 (7.2) | |
| IIA | 1 (1.3) | 12 (7.9) | |
| IIB | 3 (4.0) | 7 (4.6) | |
| IIIA | 13 (17.5) | 24 (15.8) | |
| IIIB | 11 (14.8) | 20 (13.2) | |
| IV | 34 (45.9) | 64 (42.3) | |
| Grouped by stages | n, % | n, % | .27 |
| Early (IA, IB, IIA, IIB) | 16 (21.6) | 43 (28.4) | |
| Advanced (IIIA, IIIB, IV) | 58 (78.3) | 108 (71.5) | |
| Histologic type | n, % | n, % | |
| Squamous | 26 (35.1) | 44 (29.1) | |
| Adenocarcinoma | 21 (28.3) | 50 (33.1) | |
| Small cell | 8 (10.8) | 34 (22.5) | |
| Large cell | 1 (1.3) | 6 (3.9) | |
| Non-small-cell lung carcinoma | 18 (24.3) | 17 (11.2) | |
LCSP: patients evaluated in the rapid-access lung cancer screening program; non-LCSP: patients who were not seen in the LCSP; DE: standard deviation; PET: positron emission tomography; EBUS-TBNA: real-time endobronchial ultrasound-guided transbronchial fine-needle aspiration.
The comparison of the therapeutic measures adopted, as well as the delays in different diagnostic–therapeutic intervals, is demonstrated in Table 4. As for the delays in carrying out the different diagnostic tests, there were only differences in favor of the LCSP group for performing EBUS-TBNA. We found no differences in the therapeutic delays, and the majority of the patients received oncologic treatment with chemotherapy either alone or associated with radiotherapy (64.8% vs 72%); less than one-fourth of the patients in both groups underwent surgical resection (24.3% vs 22.3%) and, in each, one patient refused any type of therapy. There were also no differences in the time intervals transpired between the date of the first assessment and the TNMc staging of the tumor, nor between the staging and the start of treatment. The same result was obtained by comparing the intervals between the date of the derivation and the TNMc staging, and between the staging and the start of treatment.
Therapeutic Measures Adopted in the LCSP and Non-LCSP and Comparison Between Both Groups of the Delays in Different Diagnostic–therapeutic Intervals.
| LCSP | Non-LCSP | P | |
| Delays in diagnostic tests in days from the first evaluation: median (interquartile range) | (n=74) | (n=151) | |
| CT-thorax | 5.0 (2–7) | 3.0 (0–7) | .19 |
| Bronchoscopy | 8 (4–15) | 10.0 (5–16) | .25 |
| FNA | 16 (7–26) | 17.0 (8–33) | .51 |
| PET | 16.0 (10–23) | 20.0 (11–28) | .14 |
| EBUS-TBNA | 17.0 (13–22) | 28.0 (16–40) | .04 |
| Therapeutic measures adopted | (n=73) | (n=150) | |
| Surgery: n, % | 18 (24.3) | 34 (22.6) | .70 |
| Oncology: n, % | 48 (64.8) | 109 (72.2) | .26 |
| Palliative treatment: n, % | 8 (10.8) | 7 (4.6) | .15 |
| Therapeutic delays in days since the first evaluation: median (interquartile range) | |||
| Interval until surgical treatment | 32.5 (21–45) | 36.0 (27–59) | .18 |
| Interval until the start date of oncologic treatment | 24 (20–35) | 22.0 (16–37) | .70 |
| Interval until the start date of palliative treatment | 35 (14–62) | 15.0 (10–29) | .22 |
| Interval in days between the first evaluation and staging: median (interquartile range) | 13.5 (7–23) | 15.0 (7–26) | .55 |
| Interval in days between the first evaluation and the start of treatment: median (interquartile range) | 29.0 (20–29) | 25.5 (18–43) | .72 |
| Interval in days between the date of referral and staging: median (interquartile range) | 20.0 (12–30) | 21.0 (10–33) | .90 |
| Interval in days between the date of tumor staging and start of treatment: median (interquartile range) | 14.7 (9–20) | 12.0 (7–18) | .28 |
LCSP: patients evaluated in the rapid-access lung cancer screening program; non-LCSP: patients who were not seen in the LCSP; chest CT: computed tomography of the chest; FNA: transthoracic fine-needle aspiration; PET: positron emission tomography; EBUS-TBNA: real-time endobronchial ultrasound-guided transbronchial fine-needle aspiration.
The mean time transpired from the date of the referral to the LCSP was 6.2 days; in contrast, the delay for an ordinary pulmonology consultation during this same time period was slightly more than 14 days.
Lastly, Table 5 compares the results related with the hospitalization data, as well as the percentages of patients who met the recommendations of the BTS Guidelines6 and the NHS Cancer Plan,7 related with diagnostic–therapeutic delays. Regarding hospitalization, in 56 patients of the LCSP group (75.6%) the entire diagnostic study was done without requiring hospitalization, which showed a significant difference over the non-LCSP group. The same was true when we compared (among those patients with hospitalization) the days of hospitalization in the pulmonology unit or the total number of hospitalization days. In the LCSP group, the average number of hospitalization days was 2, the reason in most of these cases being the CT-guided FNA.
Comparison Between the LCSP and Non-LCSP Hospitalization Data and Compliance With the Recommendations for Diagnostic–Therapeutic Delays.
| LCSP (n=74) | Non-LCSP (n=151) | P | |
| Hospitalization data | |||
| Patients who were hospitalized during the study: n, % | 18 (24.3) | 89 (58.9) | <.0001 |
| Days hospitalized in pulmonology: median (interquartile range) | 2 (2–3) | 5 (2–11) | .0005 |
| Total hospitalization daysa: median (interquartile range) | 2 (2–3) | 8 (3–13) | <.0001 |
| Evaluation of delays according to recommendations: n, % | |||
| ≤7 days between referral and SC consultationb | 63 (85.1) | 117 (77.4) | .17 |
| ≤56 days between SC consultation and surgeryb | 16 (88.8)d | 24 (70.5)d | .70 |
| ≤62 days between the referral and the start of any type of therapyc | 69 (94.5) | 128 (85.3) | .04 |
LCSP: patients evaluated in the rapid-access lung cancer screening program; non-LCSP: patients who were not seen in the LCSP; SC consultation: consultation in pulmonology or specialized care.
Although a high percentage of cases from both groups met the recommendations for the diagnostic–therapeutic delays, these percentages were always higher in the LCSP group, where more than 85% met the recommendations, which was statistically significant compared with the recommendation of the UK Department of Health to initiate treatment within 62 days.
DiscussionDespite the growing interest in making patient care in LC more efficient and faster, several international and national studies have demonstrated that the time transpired to diagnosis and treatment in this disease is longer than what is recommended in published guidelines.17–21 In Spain, this fact was the reason behind the creation of rapid-access pulmonology consultations at many health-care centers that offered ambulatory studies with similar timeframes as in the case of hospitalized patients and whose results indicate a notable reduction in delay times.10–14
In our setting, we have been able to confirm a marked underuse of the LCSP as only 26.7% of the appointments available were actually used; however, those patients who were seen in the LCSP program met with the referral protocol. More than half of the cases were neoplastic processes, and 44.5% of the correct referrals were found to be LC. This percentage is to that observed in a recent study done in Spain.14 There were very few unjustified appointment requests, many of which were probably related with the errors in the appointment service and not with improper referrals by physicians. Most patients were referred from PC (67.5%) and only 1.3% were referred from the emergency department. In contrast, in the non-LCSP groups 29.1% of the cases were sent from the emergency department. Unlike other studies, there were no remissions from the ordinary pulmonology consultations as we consider that this duplication in specialized consultations could result in an increase in delay times. As is logical, the speed in having an appointment is an important benefit of the LCSP compared with derivations to ordinary pulmonology consultations; while the mean delay time in the former was 6.2 days, in the latter the delays averaged 2 weeks.
Each year, our department diagnoses around 30% of all the LC in the region of Navarra, and in the last two years that we have analyzed, only 16.5% were evaluated in the LCSP. Therefore, it is still very frequent for patients with suspicion for LC to be remitted to another type of consultation, or rather straight to the hospital ER.
The mean age (less than 65 in both groups) is lower than that was found in national studies from some years ago, and as it was published in the review about LC in Spain written by Sánchez de Cos,3 we have verified a significant increase in adenocarcinomas and a reduction in epidermoids over what has been observed in previous studies. Overall, the percentage of patients who are subjected to surgical treatment is slightly higher than that described in these publications and, contrarily, the number of patients who only receive palliative medication is lower. Unfortunately, and as has been frequently seen in other studies, the majority of LC diagnosed by LCSP corresponded with advanced stages (45.9% stage IV) and only 21.6% were diagnosed in early phases, which is a situation that is similar to what happens in the non-LCSP group.
We have only evaluated the delays in the diagnostic tests that may entail delays in the study; other tests (work-up, lung function studies, etc.) are done without delay when there is suspicion for LC. In all the LCSP cases, helical computed tomographies (CT) were done of both the chest and upper abdomen with contrast as an initial complementary test, as it is a standard practice in our group to have a CT available before bronchoscopic exploration. Out of all the patients evaluated in the LCSP, 29.7% required FNA, which is a percentage that is similar to that found in the non-LCSP group (29.7% vs 27.1%) and, in some of these cases, it was necessary to repeat the test in order to acquire another sample. In our hospital, we carry out transthoracic FNA in cases of LC only in hospitalized patients, and this was the cause for most of the hospitalizations in the LCSP group. Approximately half of the patients studied had a PET/CT, and, although in the global comparison there were no significant differences, in the last 2 years the indication for EBUS-TBNA has been greater in the LCSP group.
In spite of the fact that the vast majority of diagnostic explorations can be done in an ambulatory setting, in Spain it is still relatively frequent to justify hospitalization of patients with LC due to the delay times.21 While we admit that there may be some differences between centers, our findings (in most cases, all the diagnostic tests were done in less than 3 weeks) demonstrate that it is possible to study LC in an ambulatory setting without entailing any inappropriate delays in the diagnosis.
As has been commented, many studies have revealed excessive delays in the diagnosis and treatment of LC.17,25 A study done in Finland showed evidence that half of the patients with LC did not meet the recommendations of the BTS, with a mean interval of 82 days between the referral from the PC physician and the start of treatment.18 Recently, in Spain Sanz-Santos et al.,14 using a rapid-access thoracic cancer diagnosis program, have achieved much better results that agree with ours. We have been able to confirm that, in the 2 groups analyzed, the mean times transpired until the clinical staging of LC and treatment (including surgery) have been within the limits of most recommendations in a high percentage of cases.
Some of the previously cited studies make reference to the time transpired until the anatomopathologic confirmation. In our study, while admitting that it may be less of a standardized interval and more dependent upon the center, we have contemplated the delays until TNMc staging since, as these are significantly greater and the TNMc is the variable that will condition the therapeutic approach in the end, we believe that this provides useful data in order to improve the clinical management of LC. Nevertheless, we completely agree with the recommendation made in a recent review about the need to adopt a standardized definition for each relevant time interval.17 The inclusion of PET and EBUS-TBNA doubtlessly could have been a determinant for lengthening the diagnostic interval, but in our case, thanks to the agreements reached with the private health-care center that performs PET and the inexistence of important delays for EBUS-TBNA, this fact has not been transcendental.
The lack of significant differences in the diagnostic delays between the two groups may be surprising. This result could have various explanations: in the first place, in the non-LCSP group the percentage of admittances by the emergency department was 29.1 and, in these cases, the referral date coincides with that of the emergency visit; in contrast, in the LCSP group this percentage was only 1.3%, and that could partially make up for the longer delay for appointments in the ordinary pulmonology consultations. On the other hand, the patients treated by non-LCSP have benefitted from the advantageous situation obtained with the commitments acquired for the LCSP. Finally, it is also possible that the health-care resources available in our province may be superior to those in other provinces.
Unfortunately, carrying out diagnostic studies and initiating therapy without delay does not mean that survival increases. In fact, in many of the published studies mentioned, the patients who received faster treatment presented poorer survival rates. In these studies, patients with advanced disease at the time of the presentation have a higher probability of having symptoms and signs of LC. Consequently, diagnosis and palliative treatment are done faster due to the earlier referral to the specialist and to the need for fewer diagnostic tests in order to evaluate the tumor.14,26 It is also more probable that these patients receive only one support treatment and die before those who present with initial-stage disease.17
Where we have found that important differences have been in the number of hospitalizations. 24.3% of the patients in the LCSP group required hospitalization compared with close to 60% of the other group, and in the cases of LCSP in which hospitalization was considered necessary, and the average stay was only 2 days. For some time, there has been a wide consensus that part of the hospital resources used in LC are inadequate, and so it was indicated at the end of the 1990s by some members of the SEPAR bronchogenic carcinoma workgroup.20 Along these lines, a study done in our country confirmed that many of the patients hospitalized due to suspicion of LC were stable when admitted and that they could have been evaluated in an outpatient setting.27
Despite the difficulties to draw conclusions when analyzing the studies about costs derived from care provided in LC, it is evident that the majority of the costs are generated by hospitalization.5 In our country, Abal et al.4 found that the mean cost for patients studied in an outpatient setting was 62% less than that generated by hospitalization. A recent Canadian publication, in which 51% of the LC patients were studied while hospitalized, demonstrated the important increase in health-care costs when patients of this disease are hospitalized, stating that the cost can be up to 7 times less in patients who are seen in an ambulatory setting.28
One limitation of our study is related with the fact that the LCSP group covers 3 years more than the non-LCSP. Although from a methodological point of view this procedure may be questionable, we have confirmed an absence of significant differences between the cases of LCSP evaluated in the first 3 and the last 2 years for both diagnostic tests ordered (except EBUS-TBNA) as well as diagnostic–therapeutic delays or data referring to hospitalization. Thus, we consider that these findings validate in some manner our approach and, in doing so, we were able to include a larger number of cases. The fact that we did not analyze the reasons for the patients remaining in the hospital in the non-LCSP group is another limitation; nonetheless, the lack of differences between the 2 groups in sociodemographic data and distribution by LC stage leads us to believe that, in many cases, hospitalization could have been avoided.
In order to correctly study this serious pathology in the ambulatory setting, it is necessary for LC tumor committees and pulmonologists involved in LC management to agree upon maximal delay times with the administration and all departments involved in LC diagnosis and treatment and to jointly design adequate strategies that adapt to each setting. Although LCSPs probably do not improve the prognosis of the disease, they undoubtedly contribute to improving the quality of life and emotional well-being of patients. Furthermore, they help avoid unnecessary hospitalizations, reducing health-care costs, which is extremely important when we consider the economic situation that our country is currently experiencing.
In conclusion, we are able to state that in our setting a rapid-access LCSP is able to perform outpatient diagnostic studies in a large percentage of cases, all within the timeframes recommended by current guidelines. Nonetheless, we have detected a striking underuse of these services, which requires us to improve the coordination among the departments that may refer these patients. This is especially true for PC, the main entry way for patients to the LCSP, and also the emergency department, where it is still very frequent for these patients to be hospitalized even if, in many cases, these patients could be correctly treated as outpatients. We should make an effort to inform the physicians of these departments and insist on how LCSPs provide advantages.
Conflict of InterestsThe authors declare having no conflict of interest.
We would like to acknowledge the work and dedication of the professionals from the Departments of Anatomic Pathology, Thoracic Surgery, Oncology and Radiodiagnosis at the Complejo Hospitalario de Navarra.
Please cite this article as: Hueto Pérez De Heredia J, et al. Evaluación de la utilización de una consulta de diagnóstico rápido de cáncer de pulmón. Tiempos de demora diagnóstica y terapéutica. Arch Bronconeumol. 2012;48:267–73.