The prevalence of chronic obstructive pulmonary disease (COPD) in Spain is 11.8%, with an underdiagnosis up to 74.7% and high morbidity and mortality rates, being one of the main three death causes worldwide.1,2 Irreversible and gradual damage to the alveoli causes emphysema, leading to impaired gas exchange, trapped air and dynamic hyperinflation, producing dyspnoea on exertion.3,4 Spanish COPD guidelines (GesEPOC) recommend searching for treatable traits in high-risk COPD patients, such as bronchoscopic lung volume reduction techniques (BLVR),3 also recommended by the GOLD Report, with evidence A for endobronchial valves (EBVs) and B for bronchoscopic thermal vapor ablation (BTVA).2 Comparation between BLVR and lung volume reduction surgery (LVRS) showed no significant differences in primary outcomes,5 and Polverino F. and Sciurba F. support starting by EBV since surgery is irreversible.6
The major limitation for using EBV is the collateral ventilation (CV) through incomplete fissures, while BTVA induces a thermal reaction causing inflammation, scarring and localised fibrosis.7,8 Our objective is to analyse the results of the use of BTVA in our patients with advanced COPD.
We collected patients treated with BTVA from June 2019 to February 2023, using the same criteria from lung volume endoscopic treatment in emphysema described in prior clinical trials.9 Intervapor® Perzonalized Procedure Program (IP3®) by Uptake Medical was used to inform on the severity of the condition, volume, heterogeneity of each lobe segment and fissure integrity. Analysis was made prior and 12 months after treatment.
55 patients were assessed to be candidates, of whom 24 met the inclusion criteria and complete spirometry analyses of 16 patients at 12 months were available, with the rest not being available for various reasons (3 deaths related to treatment, 2 deaths non-related to treatment, 2 patients without functional tests available 1 lung trasplant). Data were analysed using the IBM® SPSS® (Statistical Package for the Social Sciences) Statistics © software, version 25. Kolmogorov–Smirnov normality test was used, as were Student's t-test to compare normally distributed variables and the Wilcoxon test for non-parametric data. The statistical significance level was taken as p<0.05.
75% men with mean age 64 (range: 50–75 years), BMI 22.3 (range: 15–29). 100% had heterogeneous emphysema, with a mean severity of 52% (range: 26–82%). 75% had complete fissures<80%, 12.5% were between 80 and 95% and 12.5% were complete>95%.
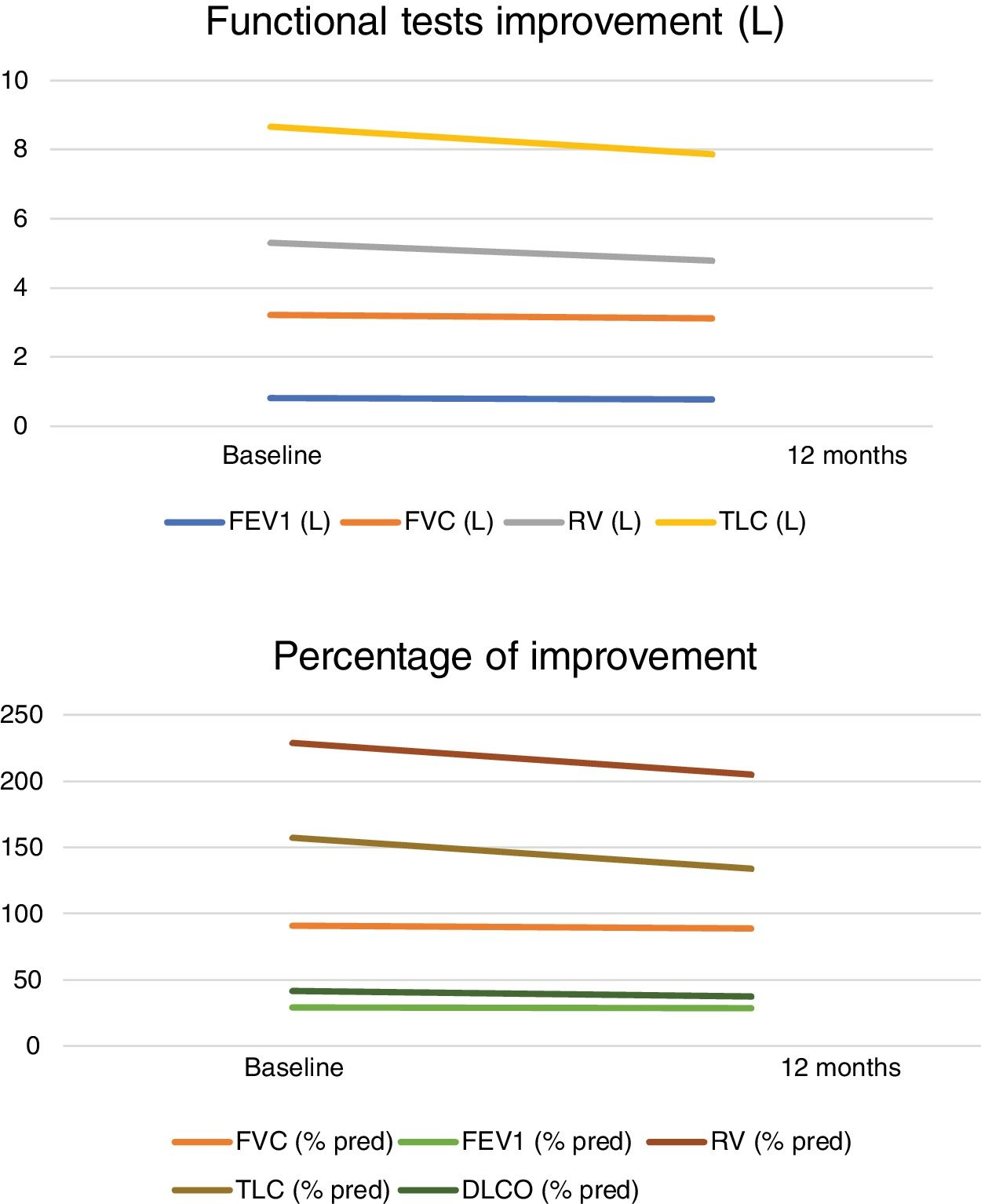
Spirometry and lung volume results before treatment: FEV1 0.819L (SD: 0.58–1.34L) percentage 29.41% (SD: 20–44%), FVC 3.22L (SD: 1.88–5.27), percentage 90.75% (SD: 69–139%), RV 5.30L (SD: 3.82–6.65L) percentage of RV of 229% (SD: 186–297%), with a TLC 8.67L (SD: 6.07–10.37L) and percentage 157.16% (SD: 110–346%), and a DLCO 41.75% (SD: 22–75%). Mean pO2 in room air blood gas samples was 78.29mmHg (SD: 65–116mmHg), and pCO2 39.49mmHg (SD: 33–46.7mmHg). The mMRC dyspnoea score was 2.83 prior to treatment (SD: 2–4).
Spirometry and lung volume results after treatment: FEV1 0.77L (SD: 0.44–1.30L) percentage of 28.58% (SD: 20–42%), FVC 3.11L (SD: 1.62–4.86), percentage of 88.91% (SD: 58–135%), RV 4.79L (SD: 3.66–6.69L) percentage of RV 205% (SD: 170–272%), with a TLC 7.86L (SD: 5.14–9.92L) and percentage 133.91% (SD: 111–185%), and a DLCO 37.25% (SD: 21–65%). The mMRC dyspnoea score was 2.42 after treatment (SD: 1–4) (Fig. 1).
Comparison of volumes at baseline and 12 months post-treatment: FEV1 (%) difference 2.88% (95% IC −2.66 to 5.65; p=0.456), FEV 1 (L) difference p=0.289, FVC difference (%) p=0.187, FVC (L) mean difference 3.42% (95% IC −0.7 to 0.38; p=0.160), TLC (L) mean difference 0.81% (95% IC −0.13 to 1.76; p=0.086), TLC comparison (%) p=0.92, RV (%) mean difference 10.5% (95% IC 4.15–44.00; p=0.022), RV (L) mean difference 9.62% (95% IC 0.19–0.73; p=0.026), DLCO mean difference 4.27% (95% CI −0.61 to 9.16; p=0.82). Dyspnoea difference prior- and post-treatment p=0.012.
The results of this study show a significant RV reduction and also a decrease on the mMRC Dyspnoea Scale at 12 months after the BTVA therapy. However, no major differences were found in the other spirometry results (FEV1, FVC, TLC) or in DLCO 12 months after treatment. Herth FJF et al.9 at 6 months observed significant differences in FEV1, and also at 12 months in the study by Gompelmann D.10 only in patients with CV. They also found significant differences in both in the Saint George's Respiratory Questionnaire (SGRQ-C) at both 6 and 12 months. However, there was no significant difference in RV at 12 months (p=0.199).10
Another aspect worth noting in this treatment is that it allows the segmental bronchi to be treated independently, or even sequentially, at different times. In this study, 20.8% were treated twice sequentially, and one patient even received three treatments.
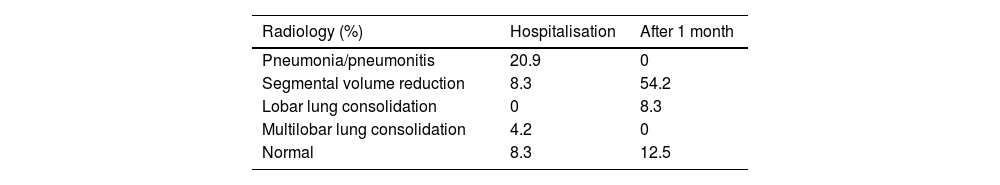
There was no incidence of pneumothorax or haemoptysis. Compared to EBV, BTVA does not produce pneumothoraces. Chest X-rays during hospital admission (for 72h) showed in 20.9% pneumonia/pneumonitis, 8.3% loss of volume, and 4.2% multilobar condensation, and the rest were normal. Giving that superinfection could not be ruled, patients were treated prophylactically with empirical broad-spectrum antibiotic treatment for 14 days and prednisolone 30mg for 3 days. However, after one month we found a loss of segmental volume in 54.2% of patients, 8.3% lobar condensations and in 12.5% there were no radiographic changes (Table 1). So, although no radiologically visible pneumonitis was observed in a certain patient group, a reduction in lung volume occurred later. Compared to the study by Herth FJF et al.,9 24% COPD exacerbation was recorded and 18% pneumonia/pneumonitis.
Results from imaging, chest X-ray from patients treated performed as inpatients in the first 72h, and later after 1 month from the treatment.
| Radiology (%) | Hospitalisation | After 1 month |
|---|---|---|
| Pneumonia/pneumonitis | 20.9 | 0 |
| Segmental volume reduction | 8.3 | 54.2 |
| Lobar lung consolidation | 0 | 8.3 |
| Multilobar lung consolidation | 4.2 | 0 |
| Normal | 8.3 | 12.5 |
Data are expressed as percentages.
Amongst all the patients studied there were 3 deaths which could be related to the BTVA treatment. One case suffered an ARDS 5 days after the treatment which required admission to intensive care unit and orotracheal intubation. Second case was admitted to hospital 2 weeks later for a COPD exacerbation and underwent several subsequent admissions with a progressive deterioration resulting in death after 85 days. The last case had COPD exacerbation at 7 days which also resulted in death. One of the patients had two segments treated in a single procedure and the other had one segment treated, never exceeding the 1700ml recommended lung volume.11
The limitation of this study is that it is a retrospective observational study, with no protocolisation and some studies are lacking such as six-minute walk test or quality of life questionnaires, but it is based on the routine clinical practice. One point we think could be improved in the study would be carrying out an IP3® lung density study after the BTVA therapy to correlate the lung volume reduction.
In summary, in the cohort studied comprising 16 patients treated with BTVA for lung volume reduction in emphysema, statistically significant and clinically meaningful differences were found in RV and on the Dyspnoea Scale after 12 months. We attribute the cause of the improvement in dyspnoea in these patients with advanced emphysema to a reduction in residual volume and therefore in hyperinflation, enhancing respiratory mechanics. However, there is very little literature on this subject and more clinical studies are necessary to support these results.
FundingThis work has not received any funding.
Conflict of interestsThe authors declare that they do not have any conflict of interest to disclosure.
We thank Alberto Herrejón Silvestre (Pulmonology, Hospital Universitario Dr. Peset, Valencia, Spain) for the help with the statistical analysis of the data.